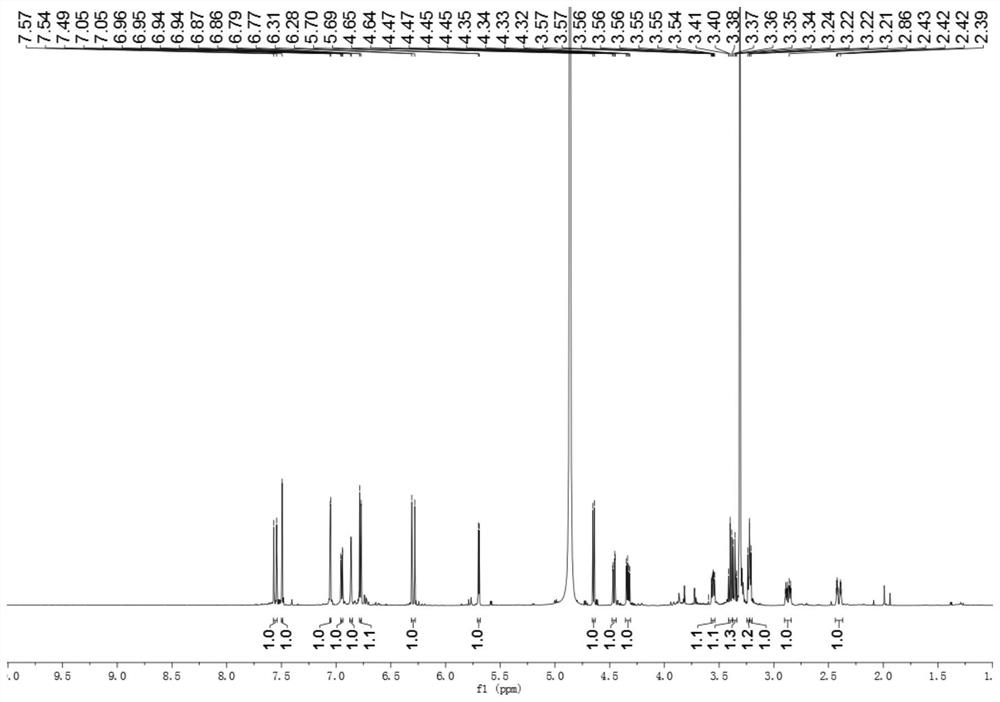
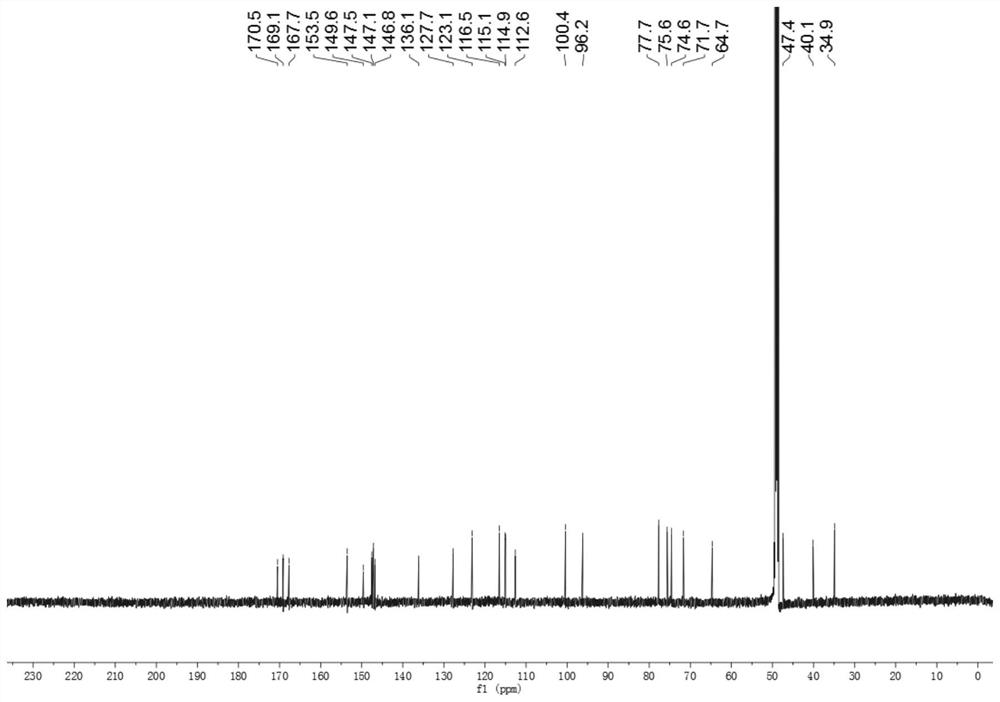
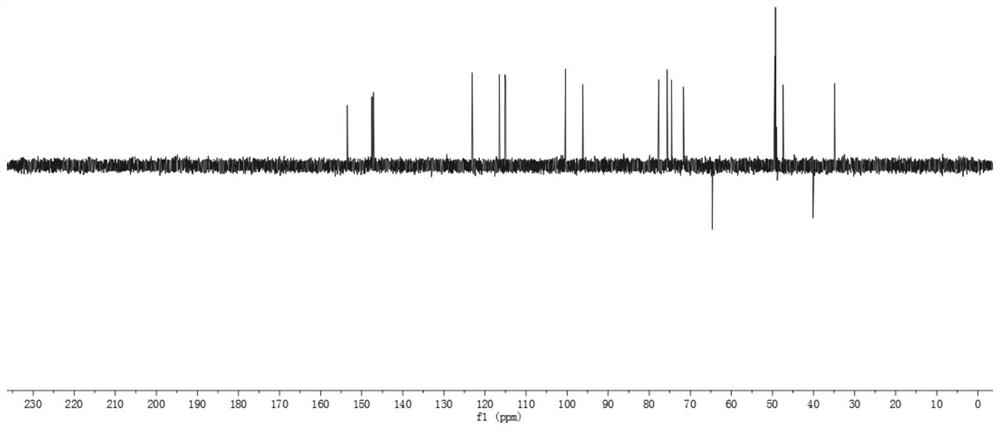
A kind of iridoid glycoside compound and its preparation method and application
A technology for iridoid glycosides and compounds, which can be applied in the field of medicine and can solve problems such as toxic and side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 compound of the present invention
[0035] (1) Take the dried medicinal material of Gardenia jasmine, extract twice with 60% ethanol under reflux for 2 hours each time, combine the extracts, and remove the solvent under reduced pressure to obtain the total extract. The total extract is dissolved in water, separated by HP-20 macroporous adsorption resin column chromatography, and eluted with water, 25% ethanol, 45% ethanol, 65% ethanol, 90% ethanol in sequence, each gradient elution has 4 column volumes (The same below), each eluate was collected separately, concentrated under reduced pressure until no alcohol smell, and obtained water elution site, 25% ethanol elution site, 45% ethanol elution site, 65% ethanol elution site, 90% ethanol elution site Elution site;
[0036] (2) Take the 25% ethanol elution part of step (1), separate through silica gel column chromatography, and use ethyl acetate-methanol-water gradient elution (100:0:0; 95...
Embodiment 2
[0038]The preparation of embodiment 2 compounds of the present invention
[0039] (1) Take the dried medicinal material of Gardenia jasmine, extract twice with 70% ethanol under reflux, each time for 2 hours, combine the extracts, remove the solvent under reduced pressure, and obtain the total extract. The total extract was dissolved in water, separated by HP-20 macroporous adsorption resin column chromatography, eluted with water, 30% ethanol, 50% ethanol, 70% ethanol, and 95% ethanol in turn, and each eluate was collected respectively, and depressurized Concentrate until there is no alcohol smell, and obtain water elution sites, 30% ethanol elution sites, 50% ethanol elution sites, 70% ethanol elution sites, and 95% ethanol elution sites;
[0040] (2) Take the 30% ethanol elution part of step (1), separate through silica gel column chromatography, and use ethyl acetate-methanol-water gradient elution (95:5:0; 90:10:0; 85:15: 0; 80:20:4; 70:30:6to60:40:8,0:100:0, v / v / v), col...
Embodiment 3
[0042] The preparation of embodiment 3 compounds of the present invention
[0043] (1) Take the dried medicinal material of Gardenia jasmine, extract twice with 50% ethanol under reflux for 2 hours each time, combine the extracts, and remove the solvent under reduced pressure to obtain the total extract. The total extract was dissolved in water, separated by HP-20 macroporous adsorption resin column chromatography, eluted with water, 35% ethanol, 55% ethanol, 75% ethanol, and 100% ethanol successively, and each eluate was collected respectively, and depressurized Concentrate until there is no alcohol smell, and obtain water elution sites, 35% ethanol elution sites, 55% ethanol elution sites, 75% ethanol elution sites, and 100% ethanol elution sites;
[0044] (2) Take the 35% ethanol elution part of step (1), separate through silica gel column chromatography, and use ethyl acetate-methanol-water gradient elution (90:10:0; 80:20:0; 75:25: 0; 70:30:4to60:40:8, v / v / v), and 8 frac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com