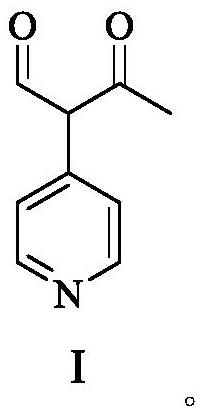
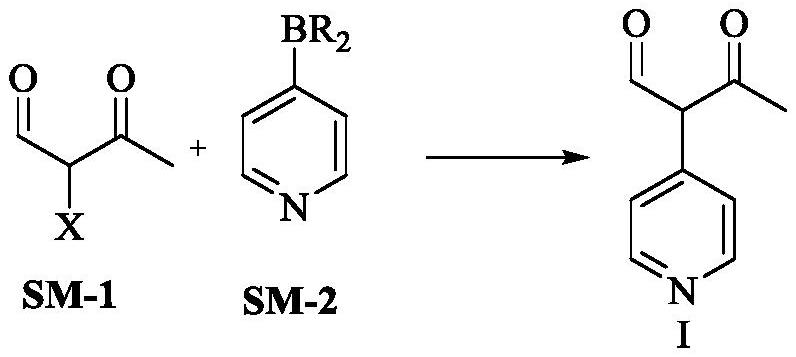
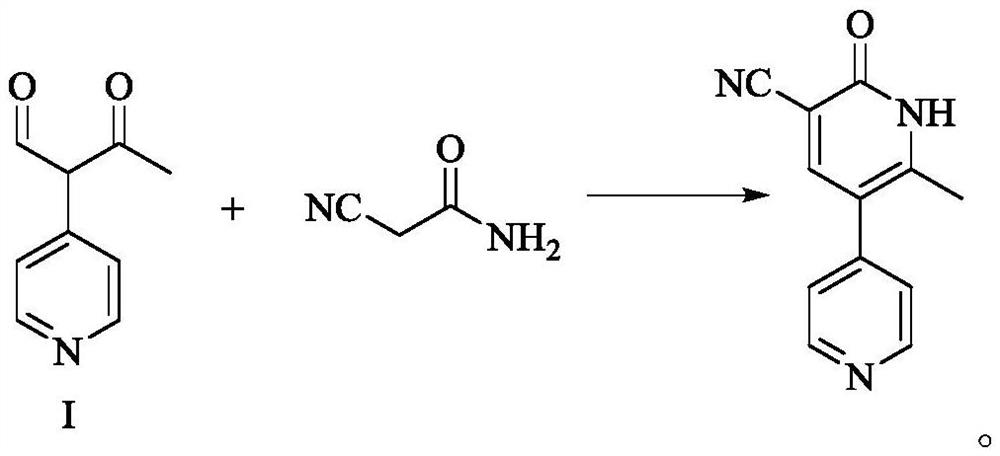
Milrinone intermediate compound
A compound, milrinone technology, applied in the field of drug synthesis, can solve the problems of low safety, low overall yield, low production cost, etc., and achieve the effects of avoiding the use of highly toxic drugs, easy route operation, and saving production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Under argon protection, at room temperature, the Pd(PPh 3 ) 4 (5.82g, 5.00mmol) was added in dimethyl sulfoxide (200mL), after stirring and mixing, a solution of KOAc (33.34g, 0.34mol) in water (50mL), α-bromoacetoacetaldehyde (SM-1X=Br, 16.48g, 0.10mol) and 4-pyridine boronic acid (SM-2R=OH, 14.74g, 0.12mol), stirring and reflux reaction, the reaction was completed, filtered, and the filtrate was added in purified water (800mL) , extracted with ethyl acetate (250mL×2), combined the organic phases, dried over anhydrous sodium sulfate, filtered, and concentrated the filtrate to dryness under reduced pressure to obtain 15.62g of milrinone intermediate compound I, with a yield of 95.8% and a purity of 99.96 %.
Embodiment 2
[0069] Under argon protection, at room temperature, the Pd(PPh 3 ) 4 (5.80g, 5.00mmol) was added in dimethyl sulfoxide (200mL), after stirring and mixing, a solution of KOAc (33.36g, 0.34mol) in water (50mL), α-bromoacetoacetaldehyde (SM-1X=Br, 16.51g, 0.10mol) and 4-pyridine boronic acid (SM-2R=OH, 12.92g, 0.105mol), stirring and reflux reaction, the reaction was completed, filtered, and the filtrate was added in purified water (800mL) , extracted with ethyl acetate (250mL×2), combined the organic phases, dried over anhydrous sodium sulfate, filtered, and concentrated the filtrate to dryness under reduced pressure to obtain 14.77g of milrinone intermediate compound I, with a yield of 90.6% and a purity of 99.92 %.
Embodiment 3
[0071] Under argon protection, at room temperature, the Pd(PPh 3 ) 4 (5.83g, 5.00mmol) was added in dimethyl sulfoxide (200mL), after stirring and mixing, a solution of KOAc (33.37g, 0.34mol) in water (50mL), α-bromoacetoacetaldehyde (SM-1X=Br, 16.52g, 0.10mol) and 4-pyridine boronic acid (SM-2R=OH, 12.27g, 0.10mol), stirring and reflux reaction, the reaction was completed, filtered, and the filtrate was added in purified water (800mL) , extracted with ethyl acetate (250mL×2), combined the organic phases, dried over anhydrous sodium sulfate, filtered, and concentrated the filtrate to dryness under reduced pressure to obtain 14.07g of milrinone intermediate compound I, with a yield of 87.5% and a purity of 99.86 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com