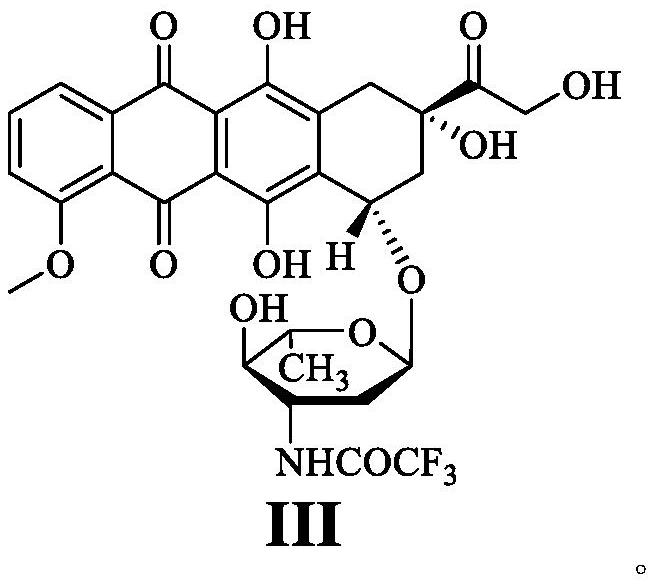
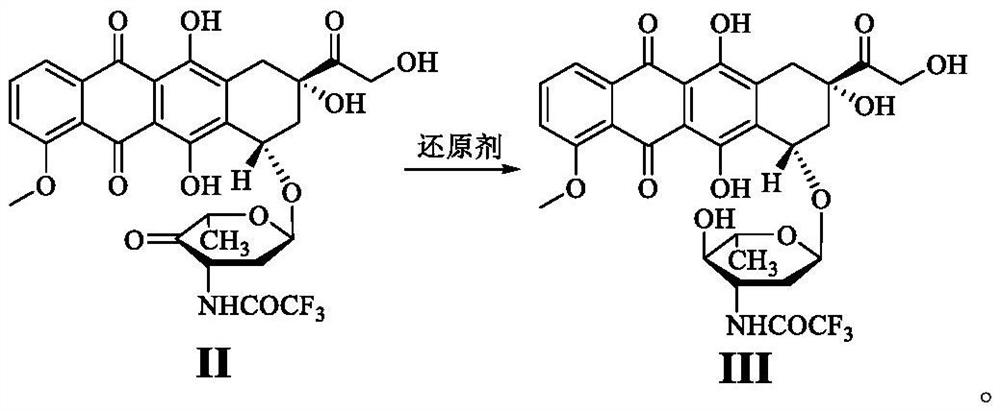
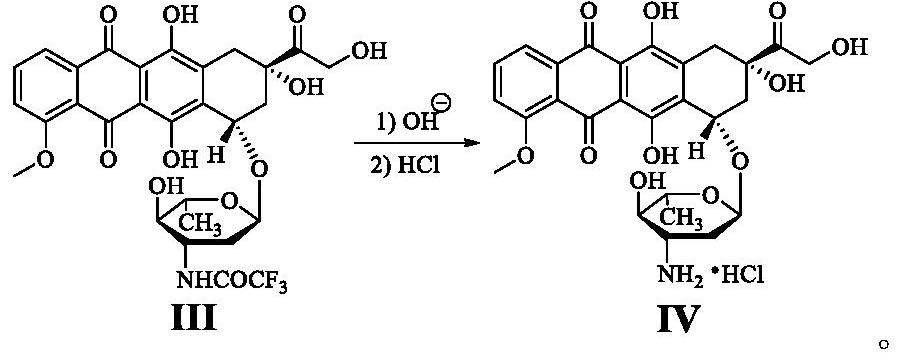
Epirubicin hydrochloride intermediate compound III
A technology of epirubicin hydrochloride and compounds, which is applied in the direction of sugar derivatives, sugar derivatives, organic chemistry, etc., can solve the problems of being unsuitable for industrial production, high production cost, and high technology requirements, so as to be suitable for industrial production and improve Safety, high-purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]Dissolve compound I (9.3g, 0.015mol) in methanol (150mL), slowly add iodobenzene diacetate (5.80g, 0.018mol), control the temperature at 0°C, stir for 1h, detect the reaction by TLC, after the reaction is complete, add ethyl acetate / Purified water (V 乙酸乙酯 :V 纯化水 =4:1, 150mL), the organic phase was collected, dried over anhydrous sodium sulfate, filtered, concentrated, crystallized by adding n-hexane (200mL), filtered, and dried to obtain compound II with a yield of 92.5% and a purity of 99.95%.
Embodiment 2
[0056] Dissolve compound I (9.3g, 0.015mol) in methanol (150mL), slowly add iodobenzene diacetate (4.83g, 0.015mol), control the temperature at 0°C, stir for 1h, detect the reaction by TLC, after the reaction is complete, add ethyl acetate / Purified water (V 乙酸乙酯 :V 纯化水 =4:1, 150mL) extraction, the organic phase was collected, dried over anhydrous sodium sulfate, filtered, concentrated, crystallized by adding n-hexane (200mL), filtered, and dried to obtain compound II with a yield of 90.4% and a purity of 99.93%.
Embodiment 3
[0058] Dissolve compound I (9.3g, 0.015mol) in methanol (150mL), slowly add iodobenzene diacetate (6.28g, 0.020mol), control the temperature at 0°C, stir for 1h, detect the reaction by TLC, after the reaction is complete, add ethyl acetate / Purified water (V 乙酸乙酯 :V 纯化水 =4:1, 150mL), the organic phase was collected, dried over anhydrous sodium sulfate, filtered, concentrated, crystallized by adding n-hexane (200mL), filtered, and dried to obtain compound II with a yield of 89.9% and a purity of 99.90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com