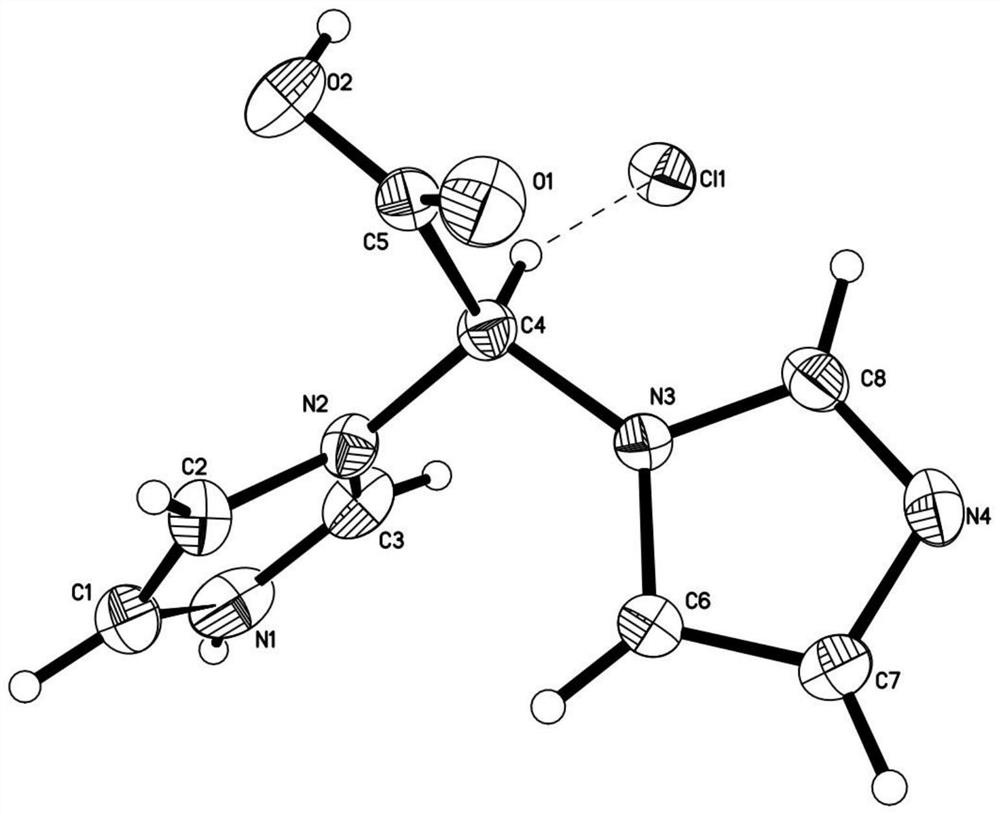
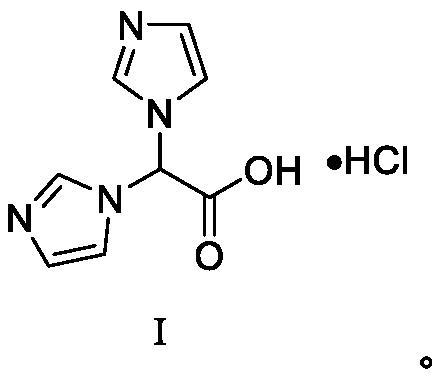
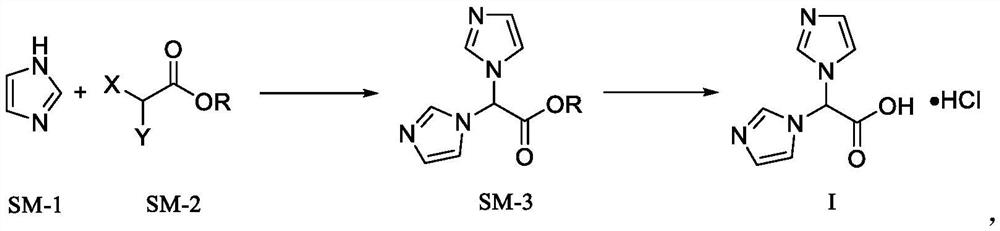
Zoledronic acid intermediate compound
A technology for zoledronic acid and compound, applied in the field of zoledronic acid intermediate compounds, can solve the problems of low environmental protection, containing bisphosphonate impurities, long reaction period and the like, and achieves improved safety, simple and controllable synthesis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Add imidazole (68.00g, 1mol), potassium carbonate (276.43g, 2mol), and tetrabutylammonium bromide (0.97g, 0.003mol) into acetonitrile (1000ml) in sequence, stir and reflux, and add ethyl dichloroacetate dropwise at the same time (54.95g, 0.35mol), after the reflux reaction is completed, filter, the filter cake is beaten and washed with acetonitrile (300ml×3), the organic phases are combined, the solvent is removed by rotary evaporation under reduced pressure, then absolute ethanol (2000ml) is added, stirred, filtered, Spin-dried to obtain 72.45g oily liquid compound 2,2-imidazole ethyl acetate, yield 93.98%;
[0058] Add concentrated hydrochloric acid (215ml) into 2,2-imidazole ethyl acetate (72.45g), stir and reflux, after the reaction is complete, spin dry, then add absolute ethanol for beating and washing (100ml×2), and dry to obtain 73.11g of white solid Compound 2,2-imidazole acetic acid hydrochloride, yield 97.21%;
[0059] Add 73.11g of 2,2-imidazole acetic acid...
Embodiment 2
[0062] Add imidazole (68.00g, 1mol), triethylamine (101.12g, 1mol), and iodine (1.27g, 0.005mol) into 1,4-dioxane (1000ml) in turn, stir and reflux, and drop dichloro Ethyl acetate (31.4g, 0.2mol), after the reflux reaction is completed, filter, filter cake is washed with acetonitrile (300ml×3), combine the organic phases, remove the solvent by rotary evaporation under reduced pressure, then add absolute ethanol (2000ml), stir , filtered, and spin-dried to obtain 39.83g oily liquid compound 2,2-imidazole ethyl acetate, yield 90.42%;
[0063] Add concentrated hydrochloric acid (80ml) into 2,2-imidazole ethyl acetate (39.83g), stir and reflux, after the reaction is complete, spin dry, then add absolute ethanol for beating and washing (100ml×2), and dry to obtain 39.92g of white solid Compound 2,2-imidazole acetic acid hydrochloride, yield 96.55%;
[0064] Add 39.92g of 2,2-imidazole acetic acid hydrochloride into 300ml of trifluoroacetic acid, add 85.55g of 85% phosphoric acid ...
Embodiment 3
[0067] Add imidazole (68.00g, 1mol), sodium carbonate (423.96, 4mol), cetyltrimethylammonium bromide (0.73g, 0.002mol) in dimethyl sulfoxide (1000ml) successively, stir and reflux, while Ethyl dichloroacetate (62.80g, 0.4mol) was added dropwise, the reflux reaction was completed, filtered, the filter cake was washed with acetonitrile (300ml×3), the organic phases were combined, the solvent was removed by rotary evaporation under reduced pressure, and then absolute ethanol ( 2000ml), stirred, filtered, and spin-dried to obtain 79.44g oily liquid compound 2,2-imidazole ethyl acetate, yield 90.17%;
[0068] Add concentrated hydrochloric acid (318ml) into 2,2-imidazole ethyl acetate (79.44g), stir and reflux, after the reaction is complete, spin dry, then add absolute ethanol for beating and washing (100ml×2), and dry to obtain 79.98g of white solid Compound 2,2-imidazole acetic acid hydrochloride, yield 96.99%;
[0069] Add 79.98g of 2,2-imidazole acetic acid hydrochloride into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com