Heat-resistant vaccine and preparation method thereof
A vaccine and heat-resistant technology, which is applied in the field of biomedicine, can solve the problems of poor heat resistance of vaccines and complex formulations of vaccine heat-resistant protective agents, and achieve good protection effects, absolute safety, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
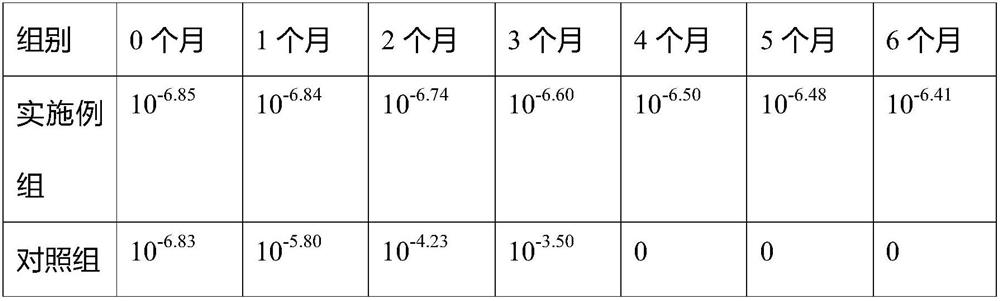
Examples
Embodiment 1
[0025] A method for preparing a heat-resistant vaccine, comprising the following steps:
[0026] S1. preparing silk globulin powder;
[0027] S2. Configure the antifreeze protection agent, wherein the solute in the antifreeze protection agent includes: the silk globulin 55g / L, lactose 100g / L, dextran 15g / L, polyvinylpyrrolidone 10g / L, glutamic acid 25g in the step S1 / L;
[0028] S3. Cool down the antifreeze protectant in step S2 to 8° C., then mix and stir the solution in step S1 and the vaccine according to the volume ratio of 1:1 for 30 minutes;
[0029] S4. Freeze-dry the mixed solution in step S3 at low temperature to obtain.
[0030] The aforementioned vaccines include live attenuated vaccines, inactivated vaccines, toxoid vaccines, vector vaccines, and edible vaccines.
[0031] The preparation method of silk globulin powder in the step S1 specifically comprises the following steps:
[0032] S11. Mix silkworm cocoons with 35-45 times the volume of deionized water, an...
Embodiment 2
[0037] A method for preparing a heat-resistant vaccine, comprising the following steps:
[0038] S1. preparing silk globulin powder;
[0039] S2. Configure the antifreeze protection agent, wherein the solute in the antifreeze protection agent includes: the silk globulin 45g / L, lactose 120g / L, dextran 40g / L, polyvinylpyrrolidone 5g / L, glutamic acid 30g in the step S1 / L;
[0040] S3. Cool down the antifreeze protectant in step S2 to 6° C., then mix and stir the solution in step S1 and the vaccine according to the volume ratio of 2:1 for 20 minutes;
[0041] S4. Freeze-dry the mixed solution in step S3 at low temperature to obtain.
[0042] The aforementioned vaccines include live attenuated vaccines, inactivated vaccines, toxoid vaccines, vector vaccines, and edible vaccines.
[0043] The preparation method of silk globulin powder in the step S1 specifically comprises the following steps:
[0044] S11. Mix silkworm cocoons with 35 times the volume of deionized water, and he...
Embodiment 3
[0049] A method for preparing a heat-resistant vaccine, comprising the following steps:
[0050] S1. preparing silk globulin powder;
[0051] S2. Configure the antifreeze protection agent, wherein the solute in the antifreeze protection agent includes: the silk globulin 50g / L, lactose 110g / L, dextran 27g / L, polyvinylpyrrolidone 7g / L, glutamic acid 27g in the step S1 / L;
[0052] S3. Cool down the antifreeze protectant in step S2 to 7° C., then mix and stir the solution in step S1 and the vaccine according to the volume ratio of 1.5:1 for 25 minutes;
[0053] S4. Freeze-dry the mixed solution in step S3 at low temperature to obtain.
[0054] The aforementioned vaccines include live attenuated vaccines, inactivated vaccines, toxoid vaccines, vector vaccines, and edible vaccines.
[0055] The preparation method of silk globulin powder in the step S1 specifically comprises the following steps:
[0056] S11. Mix silkworm cocoons with 40 times the volume of deionized water, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com