Hypoxia response chiral medicament micelle for targeting triple-negative breast cancer and preparation method of hypoxia response chiral medicament micelle
A technology for triple-negative breast cancer and chiral drugs, which is applied in the directions of drug combinations, pharmaceutical formulations, and non-active ingredients medical preparations to achieve the effects of improving drug utilization, bioavailability, and anti-tumor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
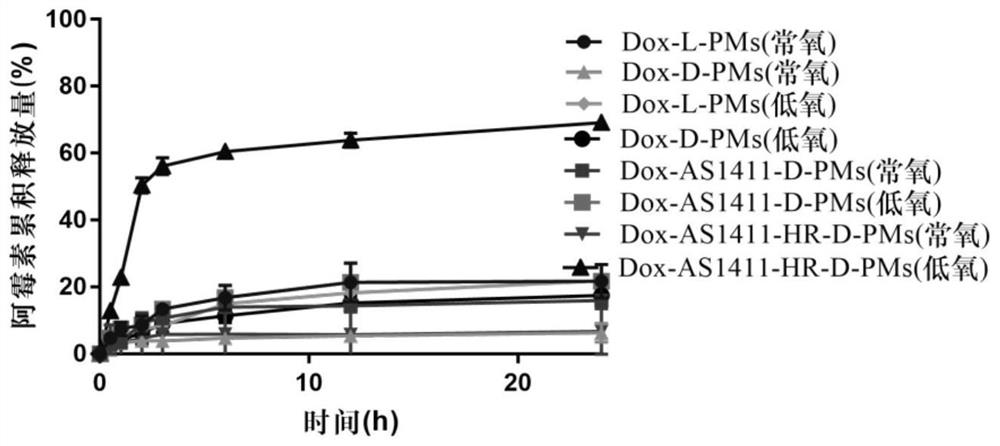
Examples
Embodiment 1-1
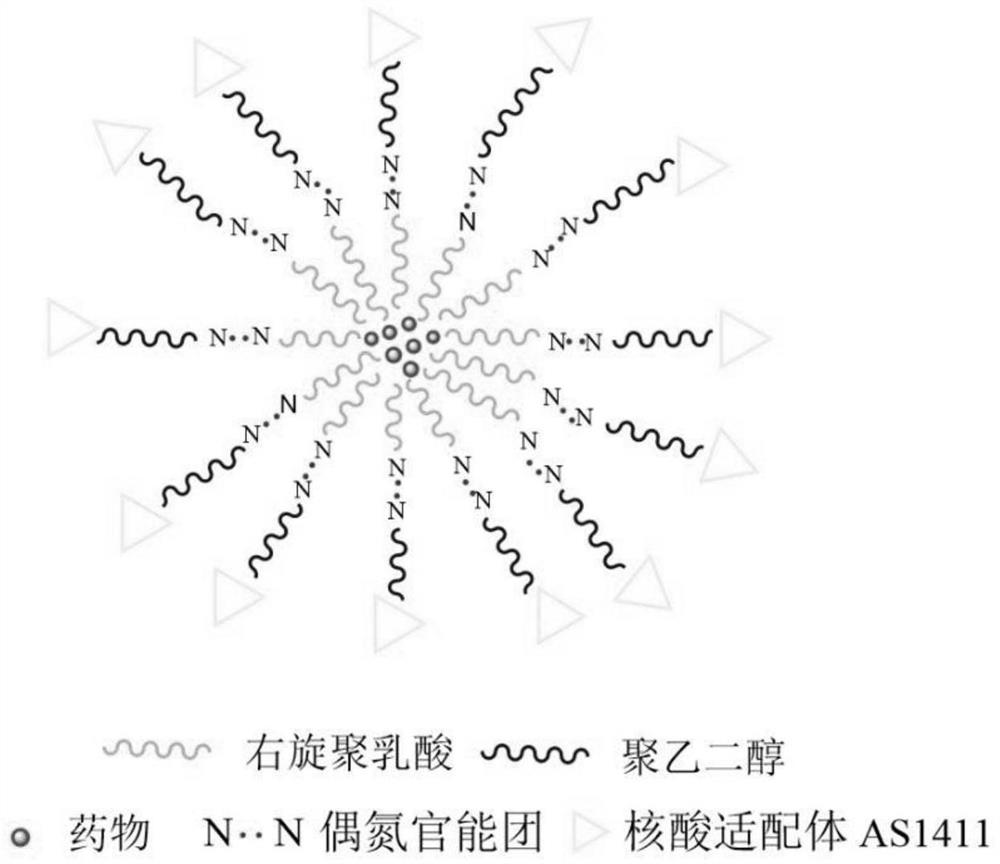
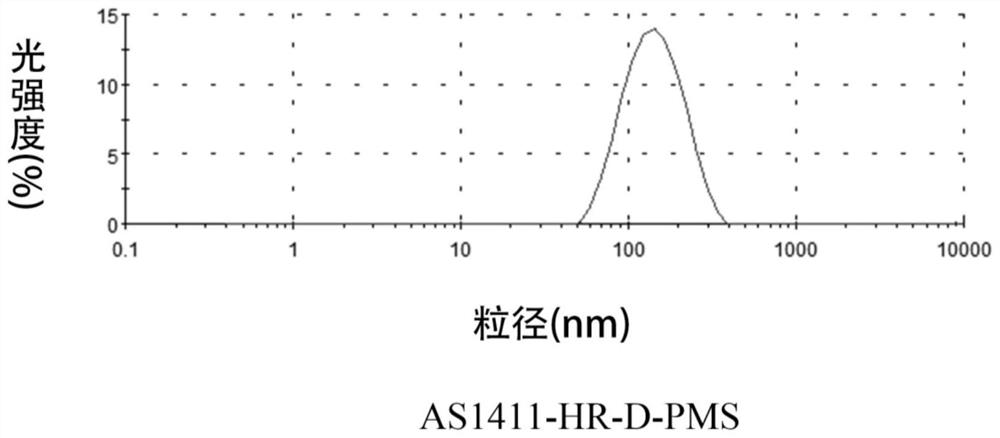
[0037] Preparation of AS1411 modified polyethylene glycol-azobenzene-dextrorotary polylactic acid block copolymer:
[0038] (1) Dissolve 50 mg (1 equivalent) of carboxypolyethylene glycol active ester (COOH-PEG-NHS, MW=1000) in 6 mL of dichloromethane, and add 6.8 mg (1.3 equivalents) of 4,4-azobis Aniline (AZO), after reacting for 24 hours at room temperature under stirring in the dark, concentrate the reaction solution obtained from the reaction, add 25mL of glacial ether for purification, let it stand overnight and then centrifuge, and dry the precipitate obtained by centrifugation to obtain carboxypolyethylene glycol azobenzene (COOH-PEG-AZO-NH 2 );
[0039] (2) Dissolve 50 mg (1.3 equivalents) of carboxy-dextrorotary polylactic acid (COOH-PDLA, MW=2000) in 5 mL of dichloromethane, and add 8 mg (2 equivalents) of dicyclohexylcarbodiimide (DCC) and 5 mg ( 2.3 equivalents) of N-hydroxysuccinimide (NHS), after stirring for 30 minutes at room temperature, add 41 mg (1 equiva...
Embodiment 1-2
[0042] Preparation of AS1411 modified polyethylene glycol-azobenzene-dextrorotary polylactic acid block copolymer:
[0043] Carboxylated polyethylene glycol active ester (COOH-PEG-NHS, MW=1000) in step (1) of Example 1-1 was replaced by carboxylated polyethylene glycol active ester (COOH-PEG-NHS, MW=2000) , and then prepare AS1411-modified polyethylene glycol-azobenzene-dextrorotatory polylactic acid block copolymer (AS1411-PEG-AZO-PDLA) according to step (2) and step (3) of Example 1-1.
Embodiment 1-3
[0045] Preparation of AS1411 modified polyethylene glycol-azobenzene-dextrorotary polylactic acid block copolymer:
[0046] Carboxylated polyethylene glycol active ester (COOH-PEG-NHS, MW=1000) in step (1) of Example 1-1 was replaced by carboxylated polyethylene glycol active ester (COOH-PEG-NHS, MW=5000) , and then prepare AS1411-modified polyethylene glycol-azobenzene-dextrorotatory polylactic acid block copolymer (AS1411-PEG-AZO-PDLA) according to step (2) and step (3) of Example 1-1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com