Novel coronavirus and culture method thereof, and novel coronavirus inactivated vaccine
A technology of coronavirus and culture method, which is applied in the field of vaccines to achieve the effect of fast value-added, high culture titer and good passage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
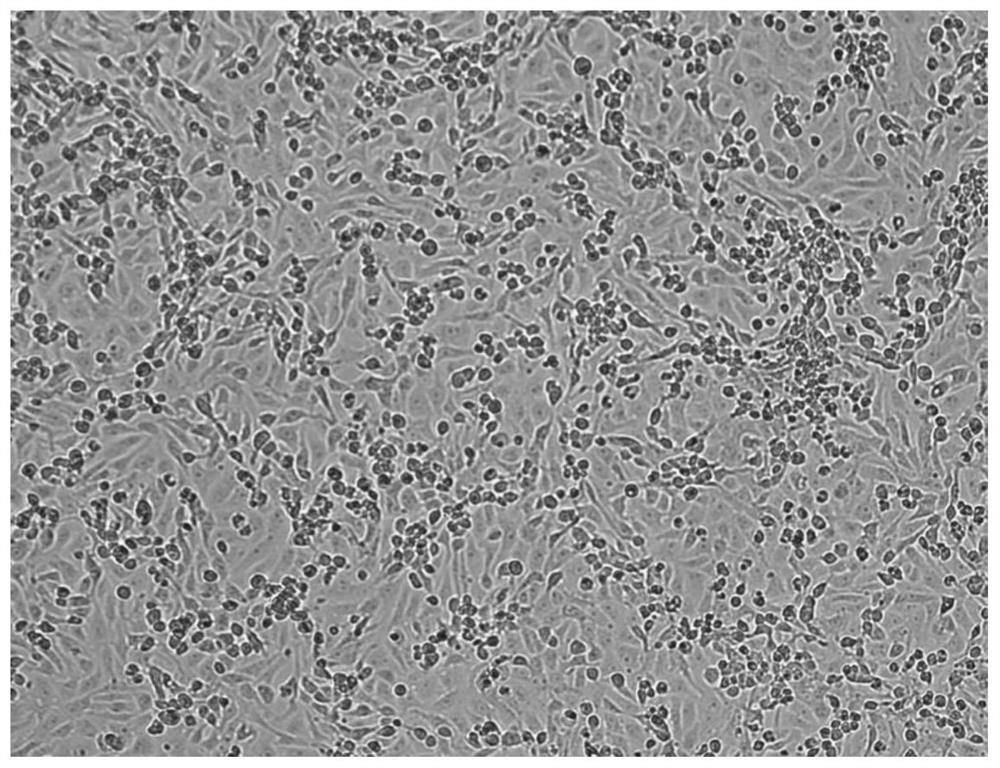
Image
Examples
Embodiment 1
[0069] Isolation, cultivation and identification of embodiment 1 K-T13 strain
[0070] 1. Sample processing
[0071] The K-T13 strain claimed in the present invention is isolated from samples of patients infected with the new coronavirus.
[0072] In a biosafety level 3 laboratory, the specimens are handled accordingly:
[0073] Add appropriate amount of MEM to the sputum bottle of the sputum sample submitted for inspection by a patient infected with the new coronavirus, vortex for 2 to 3 minutes to fully homogenize the sputum, and place it at room temperature. If no antibiotics were added, directly add double antibodies to a final concentration of 1000U / mL, and transfer to cryopreservation tubes.
[0074] Second, the configuration of the experimental solution
[0075]
[0076] Remarks: If the MEM medium contains glutamine, it can no longer be added; if the MEM medium does not contain glutamine, it is necessary to add a 1% (V / V) concentration of 200mM glutamine solution....
Embodiment 2
[0103] Embodiment 2 New coronavirus inactivation
[0104] (1) Virus harvest solution (37°C when harvested), equilibrate to 2-8°C before adding inactivator;
[0105] (2) β-propiolactone (stored at -20°C), put it on ice during use to ensure the temperature is below 2-8°C, and quickly return it to -20°C after use;
[0106] (3) Add the inactivator β-propiolactone into the virus harvesting solution (2-8°C) in different proportions, shake it evenly, and transfer it to a new storage bottle with a sterile pipette;
[0107] (4) Set up a negative control, add the inactivator (β-propiolactone) into the cell maintenance solution according to different proportions, shake it evenly, and transfer it to a new storage tube with a sterile pipette;
[0108] (5) Place the sample after adding the inactivator at 2-8°C for inactivation, and shake it once at a certain interval, and the frequency is higher at the beginning;
[0109] (6) After an appropriate time of inactivation, perform a 37°C water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com