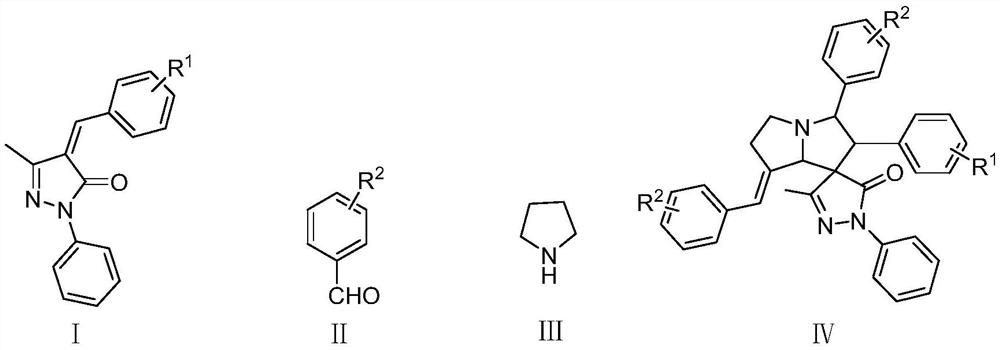
Method for synthesizing (E)-benzylidene spiropyrazole pyrrolizinone compound
A benzylidene spiropyrazole pyrroleazinone and compound technology are applied in the field of organic synthesis and can solve the problems of single compound type and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] To prepare (E)-7'-benzylidene-3-methyl-1,2',3'-triphenyl-2',3',5',6',7',7a' with the following structural formula - Hexahydrospiro[pyrazole-4,1'-pyrrolazin]-5(1H)-ketone is an example, and its preparation method is as follows:
[0029]
[0030] Add 0.1311g (0.5mmol) 4-benzylidene-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, 0.1590g (1.5mmol ) benzaldehyde, 0.0710g (1mmol) tetrahydropyrrole, then add 15mL toluene, then add 0.1200g (1mmol) acetic acid, react under the condition of heating and reflux for 14 hours, after the reaction is finished, the solvent is evaporated and concentrated, and the column is carried out by using a silica gel column. Pure (E)-7'-benzylidene-3-methyl-1,2',3'-triphenyl-2',3',5',6',7' can be obtained by chromatographic purification ,7a'-hexahydrospiro[pyrazole-4,1'-pyrrolazin]-5(1H)-one, its isolated yield is 58%, and its structural characterization data are as follows:
[0031] 1 H NMR (400MHz, CDCl 3 )δ: 7.46(d, J=7.6Hz, 3H, ArH), 7....
Embodiment 2
[0033] To prepare (E)-7'-(4-methylbenzylidene)-2',3'-bis(4-methylphenyl)-3-methyl-1-phenyl-2' with the following structural formula ,3',5',6',7',7a'-hexahydrospiro[pyrazole-4,1'-pyrrolazin]-5-(1H)-one as an example, its preparation method is as follows:
[0034]
[0035] In Example 1, the 4-benzylidene-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one used was used with an equimolar amount of 4-(4-methyl benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one replacement, the benzaldehyde used is replaced with p-tolualdehyde in an equimolar amount, the catalyst Acetic acid was replaced with p-toluenesulfonic acid, the dosage was 1mmol, and the other steps were the same as in Example 1 to obtain (E)-7'-(4-methylbenzylidene)-2',3'-bis(4-methylbenzene base)-3-methyl-1-phenyl-2',3',5',6',7',7a'-hexahydrospiro[pyrazole-4,1'-pyrrolizine]-5-( 1H)-ketone, its isolated yield is 10%, and the structural characterization data are as follows:
[0036] 1 H NMR (400MHz, CDCl 3 )δ:7.49...
Embodiment 3
[0038] To prepare (E)-7'-(2-methylbenzylidene)-2',3'-bis(2-methylphenyl)-3-methyl-1-phenyl-2' with the following structural formula ,3',5',6',7',7a'-hexahydrospiro[pyrazole-4,1'-pyrrolazin]-5-(1H)-one as an example, its preparation method is as follows:
[0039]
[0040] In Example 1, the 4-benzylidene-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one used was used with an equimolar amount of 4-(2-methyl 1.5 mmol of benzaldehyde used was replaced with 1 mmol of 2-methylbenzaldehyde, Reaction under the condition of heating and reflux for 16 hours, other steps are the same as Example 1, to obtain (E)-7'-(2-methylbenzylidene)-2',3'-bis(2-methylphenyl) -3-Methyl-1-phenyl-2',3',5',6',7',7a'-hexahydrospiro[pyrazole-4,1'-pyrrolizine]-5-(1H) -ketone, its isolated yield is 50%, and the structural characterization data are as follows:
[0041] 1 H NMR (400MHz, CDCl 3 )δ:7.49(d,J=7.6Hz,2H,ArH),7.30-7.27(m,1H,ArH), 7.25-7.22(m,2H,ArH),7.12-7.08(m,4H,ArH), 7.06-7.03(m,4H,ArH),7.01-6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com