Compound, organic electronic light-emitting device containing compound and application thereof
A compound, electromechanical technology, applied in the field of compounds, can solve problems such as the inability to meet the performance requirements of OLED devices, and achieve the effects of reducing driving voltage, improving luminous efficiency, and prolonging service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
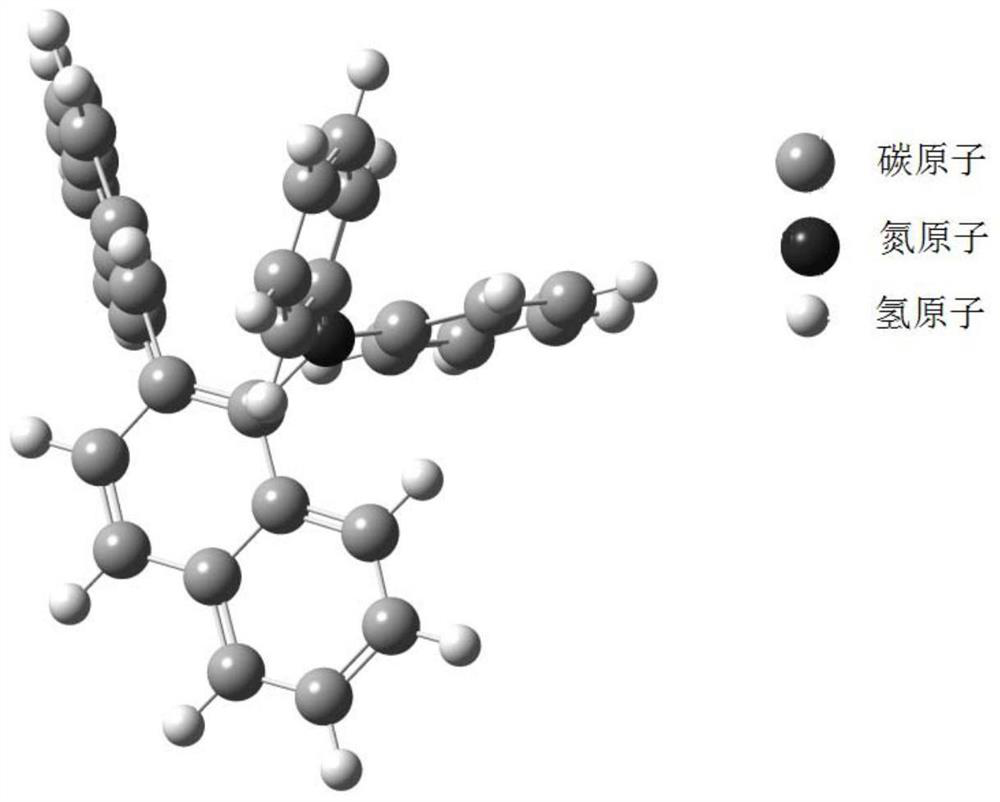
[0100] Synthesis Example 1: Synthesis of Compound P1
[0101]
[0102] In a 1000ml single-necked bottle, add 13.5g (50mmol) 1-amino-2,1'-binaphthalene, 15.7g (100mmol) bromobenzene, 0.9g (1mmol) tris(dibenzylideneacetone) dipalladium (ie Pd 2 (dba) 3 ), 0.5mL tri-tert-butylphosphine ((t-Bu) 3 P), 500ml of toluene (Toluene), 14.4g (150mmol) of sodium tert-butoxide (NaOBu-t), evacuated and changed nitrogen for 3 times, and the reaction temperature was raised to 110° C. for 5 hours. After the reaction is complete, stop the reaction. Cool to room temperature, separate the reaction liquid, concentrate the organic phase, add methanol and stir for 1 h, and filter with suction to obtain light yellow powder P1, M / Z theoretical value: 421, M / Z measured value: 422.
Synthetic example 2
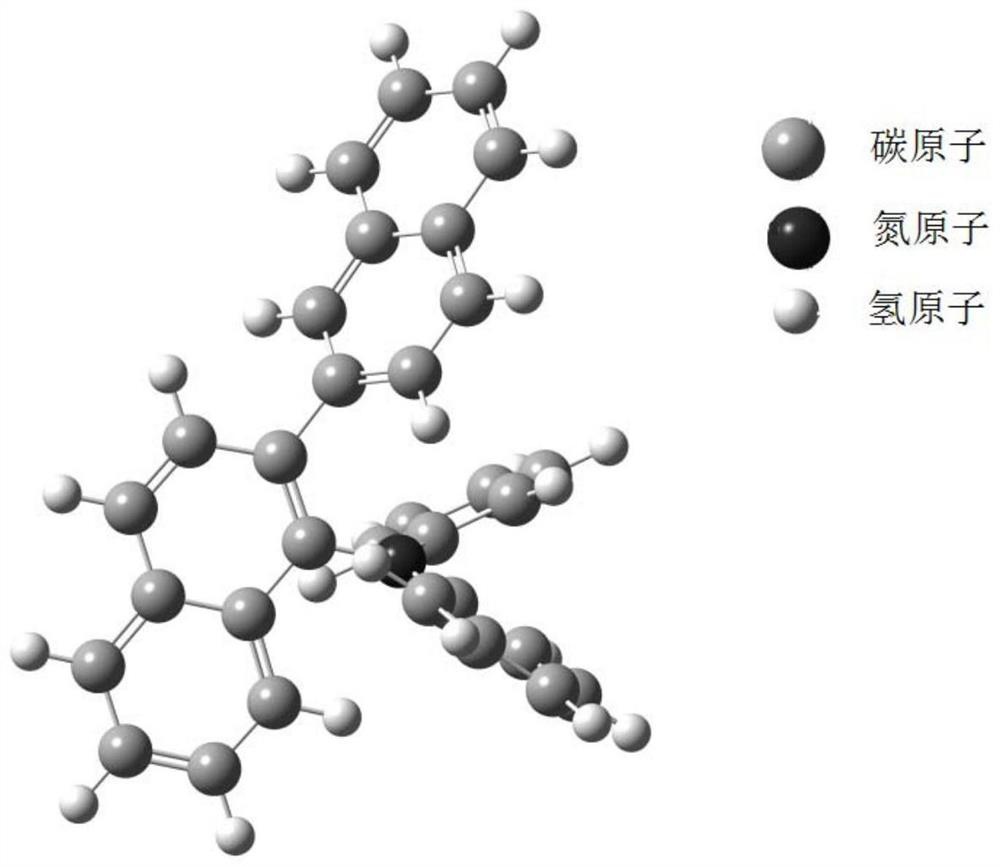
[0103] Synthesis Example 2: Synthesis of Compound P7
[0104]
[0105] In a 1000ml single-necked bottle, add 13.5g (50mmol) 1-amino-2,1'-binaphthyl, 18.7g (100mmol) 4-methoxybromobenzene, 0.9g (1mmol) tris (dibenzylidene acetone) Dipalladium (i.e. Pd 2 (dba) 3 ), 0.5mL tri-tert-butylphosphine ((t-Bu) 3 P), 500ml of toluene (Toluene), 14.4g (150mmol) of sodium tert-butoxide (NaOBu-t), evacuated and changed nitrogen for 3 times, and the reaction temperature was raised to 110° C. for 5 hours. After the reaction is complete, stop the reaction. Cool to room temperature, separate the reaction solution, concentrate the organic phase, add methanol and stir for 1 h, and filter with suction to obtain light yellow powder P7, M / Z theoretical value: 481, M / Z measured value: 482.
Synthetic example 3
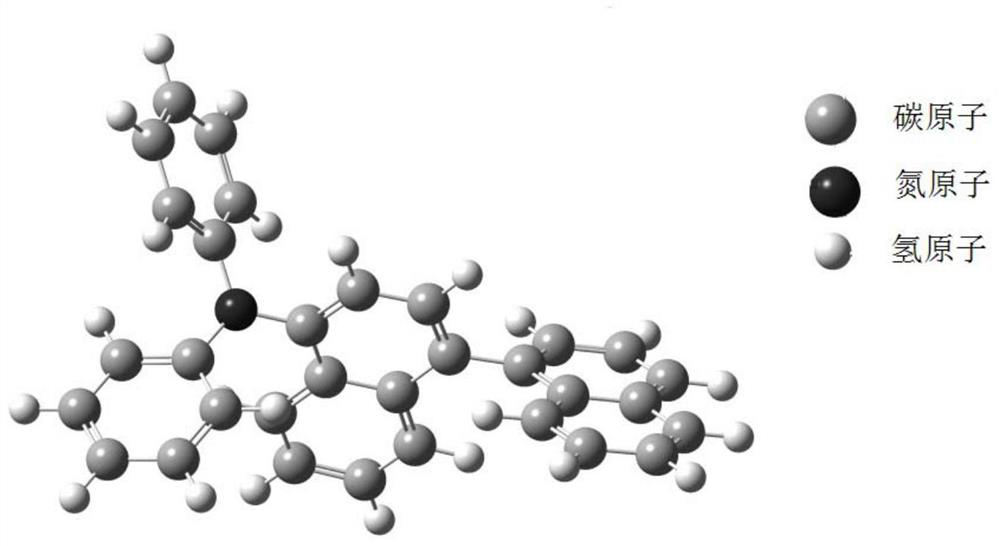
[0106] Synthesis Example 3: Synthesis of Compound P39
[0107]
[0108] In a 1000ml one-port bottle, add 13.5g (50mmol) 1-amino-2,1'-binaphthyl, 23.1g (100mmol) 4-phenylbromobenzene, 0.9g (1mmol) tris(dibenzylideneacetone) di Palladium (i.e. Pd 2 (dba) 3 ), 0.5mL tri-tert-butylphosphine ((t-Bu) 3 P), 500ml of toluene (Toluene), 14.4g (150mmol) of sodium tert-butoxide (NaOBu-t), evacuated and changed nitrogen for 3 times, and the reaction temperature was raised to 110° C. for 5 hours. After the reaction is complete, stop the reaction. Cool to room temperature, separate the reaction liquid, concentrate the organic phase, add methanol and stir for 1 h, and filter with suction to obtain light yellow powder P39, M / Z theoretical value: 573, M / Z measured value: 574.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com