Pharmaceutical application of bicyclic cyclic hexapeptide glycoside compound
A technology for peptide glycosides and compounds, applied in the field of medicine, can solve the problems of no bicyclic hexapeptide glycosides, etc., and achieve the effect of being beneficial to research and development, production, controllability and reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]The preparation method of Rubiaceae bicyclic hexapeptide glycoside cyclic peptide compound RA-XII refers to patent 201410133237.7, specifically: take the rhizome of the Rubiaceae Chinese herbal medicine Rubia yunnanensis Diels, after drying and crushing, use methanol heat reflux to extract 3 times, each time is 3-4 hours, the extract is concentrated under reduced pressure to obtain methanol extract; :3, 1:1, 0:100) gradient elution, combined the fractions by cyclic peptide TLC detection method to obtain the total cyclic peptide site; each of the following steps must be separated and purified in combination with cyclic peptide TLC detection method; the total cyclic peptide The peptide parts were chromatographed on a silica gel column, eluted with a gradient of 70:1-8:2 chloroform / methanol, and combined into three fractions Fr.1-Fr.3 according to the different cyclic peptide points; Silica gel column chromatography, 10:1-8:2 ethyl acetate / methanol for gradient elution, com...
Embodiment 2
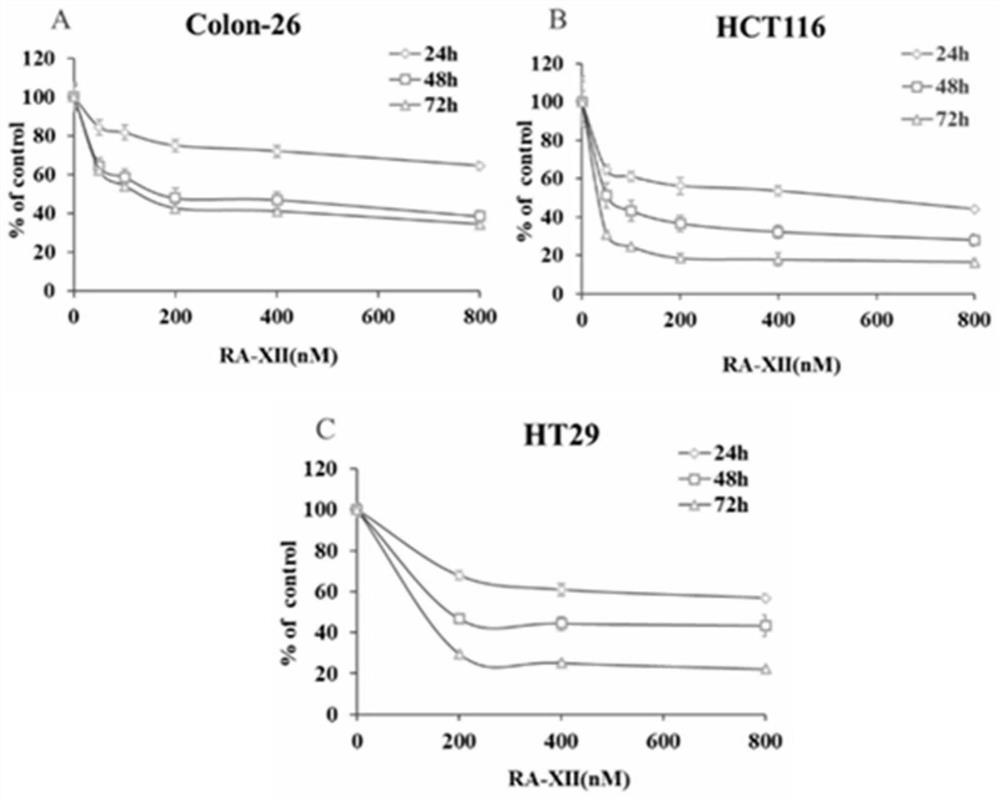
[0028] Evaluation of bicyclic cyclohexapeptide glycoside compound RA-XII on human colorectal cancer cell lines (HCT116, HT29) and mouse colorectal cancer cell line (Colon-26) and other three kinds of colorectal cancer tumor cells by MTT colorimetry The cytotoxic activity of strains, found that bicyclic cyclohexapeptide glycosides have cytotoxic activity. The experimental principles, methods and results are as follows:
[0029] Experimental principle: MTT (thiazolium blue), succinate dehydrogenase in the mitochondria of living cells can reduce exogenous MTT to water-insoluble blue-purple crystalline formazan (Formazan) and deposit in cells, while dead cells do not have this function . Dimethyl sulfoxide (DMSO) can dissolve formazan in cells, and its light absorption value is measured at a wavelength of 540nm with a microplate reader. Within a certain range of cell numbers, the amount of MTT crystal formation is proportional to the number of cells. According to the measured ab...
Embodiment 3
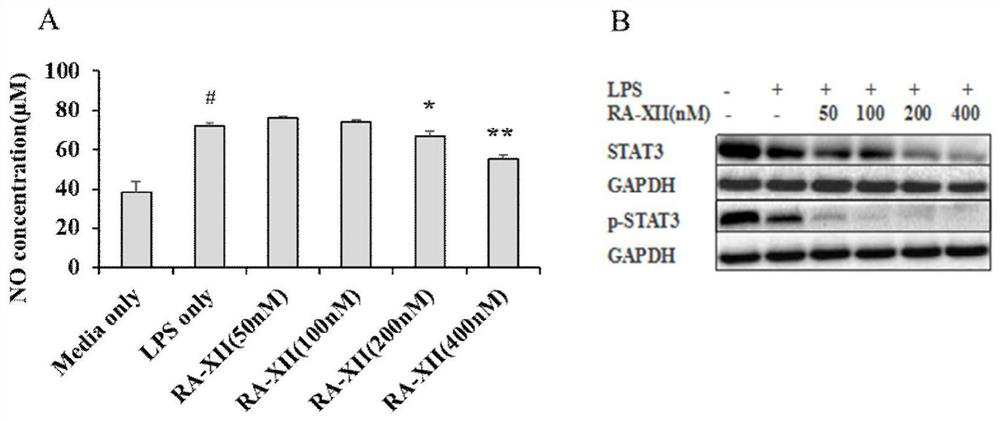
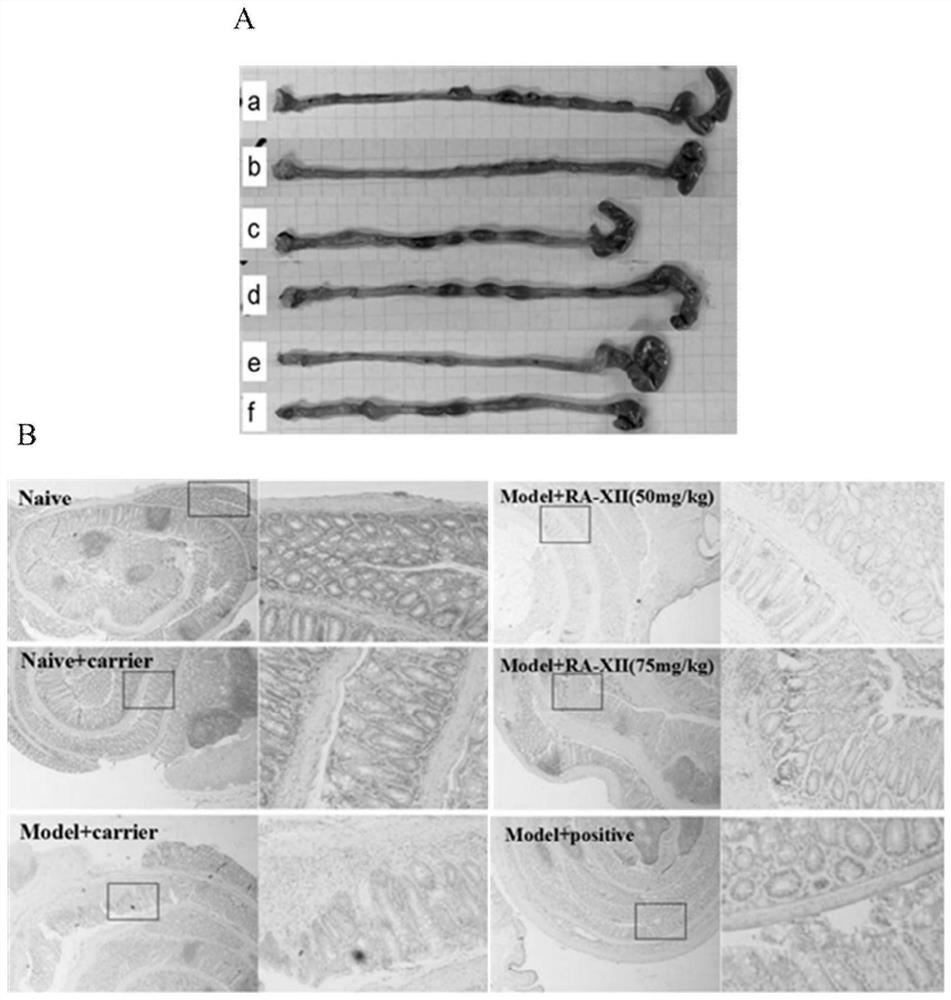
[0036] Using the co-culture model of human colorectal cancer cells HCT116 and mouse immune cells RAW264.7, the anti-inflammatory and anti-tumor activities of the bicyclic hexapeptide glycoside compound RA-XII were evaluated in vitro, and the results showed that the bicyclic cyclohexapeptide glycoside compound RA-XII had Good in vitro anti-inflammatory and anti-tumor activity.
[0037] The experimental methods and results are as follows:
[0038] RAW264.7 cells were plated in 6-well plates, 1x 10 per well 6 cells, HCT116 cells were plated in the upper chamber of a transwell 6-well plate, 5x 10 per well 5 cells, 37°C, 5% CO 2 After culturing for 24 hours, place the upper chamber of the transwell in a 6-well plate with RAW264.7, add the medium containing LPS (1 μg / ml) to make the cells produce an acute inflammatory reaction, and add the medium containing LPS (1 μg / ml) to the other groups respectively. ) and RA-XII (50nM, 100nM, 200nM and 400nM) medium, 37°C, 5% CO 2 Incubate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com