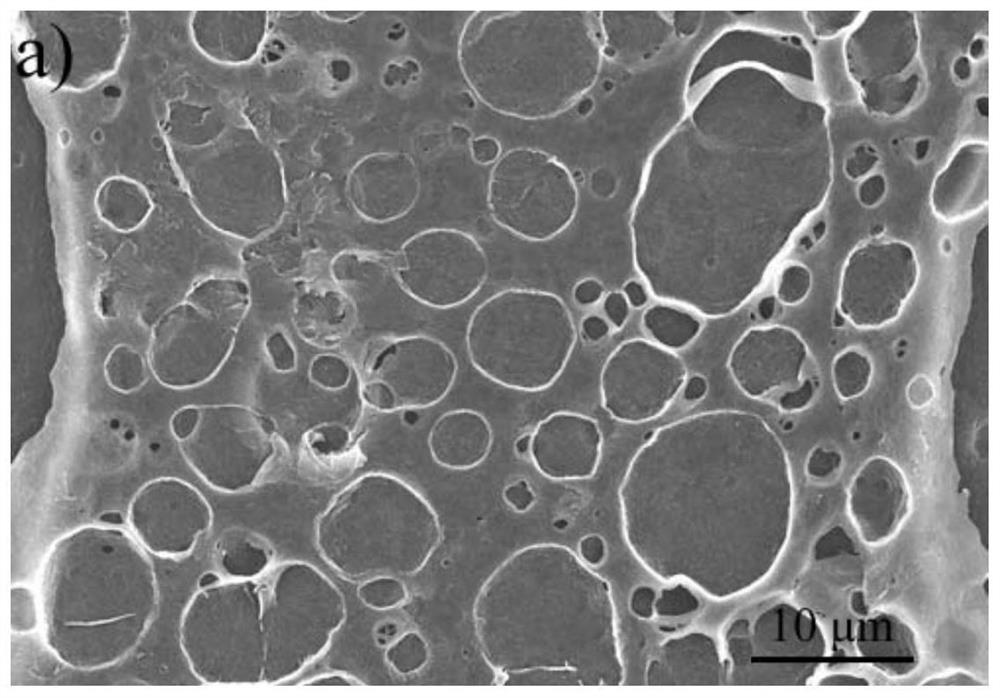
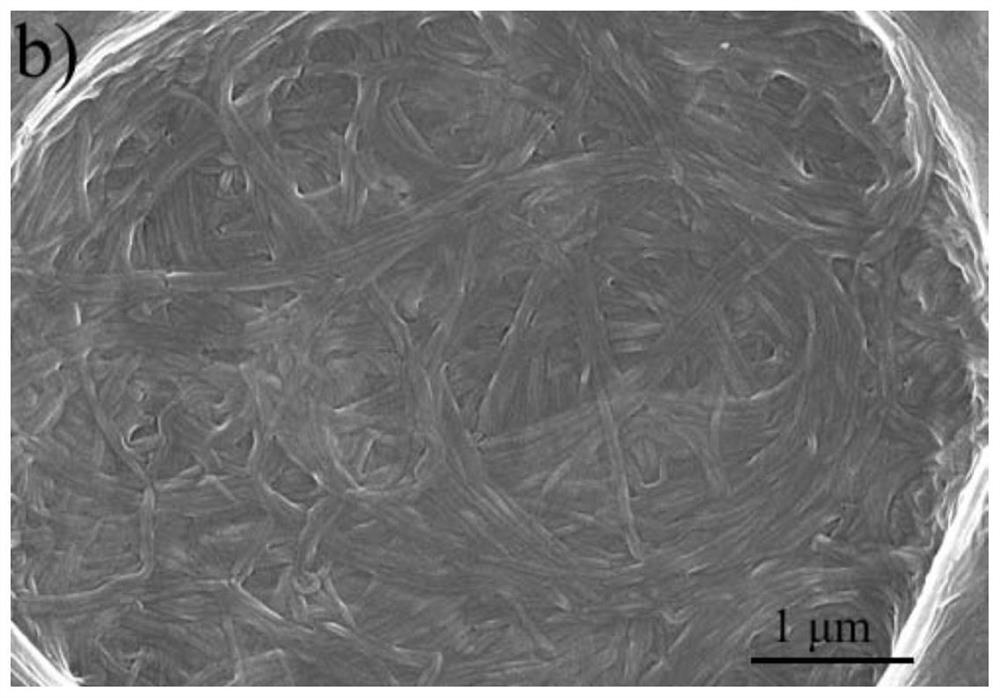
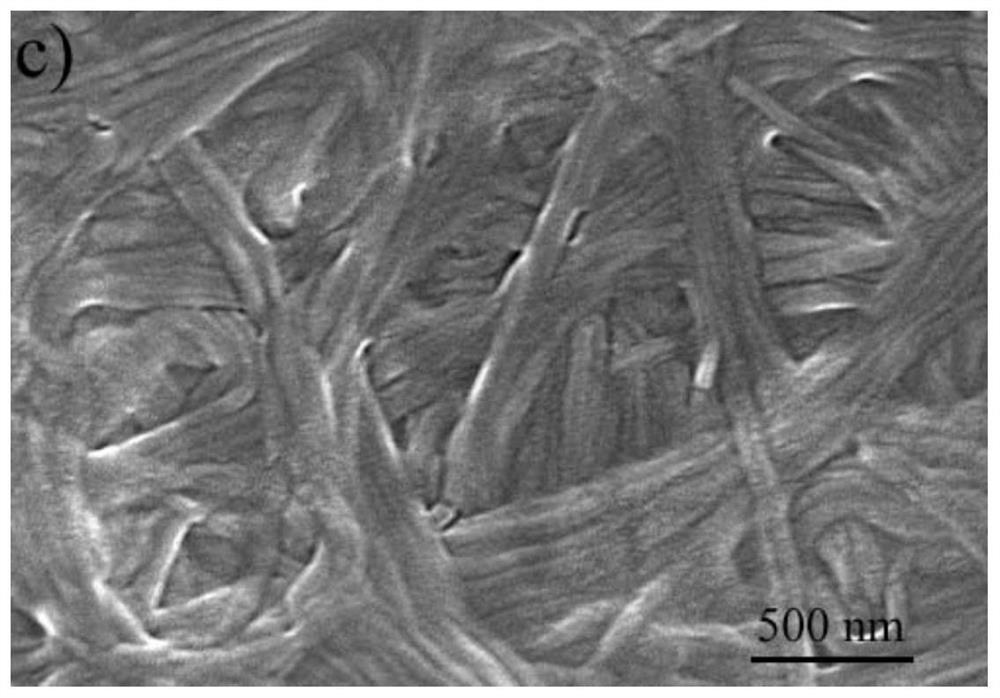
Salicylaldehyde hydrazine AIE compound, preparation method thereof, and application to construction of high-luminous-efficiency xerogel sensing film
A high luminous efficiency, sensing thin film technology, applied in the field of supramolecular fluorescence detection, can solve the problems of low luminous efficiency of xerogel sensing thin film, late start of xerogel sensing thin film material, affecting sensing performance, etc. , to achieve the effects of large-scale and cheap preparation, reduction of industrial costs, and improvement of luminous intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: Synthetic preparation of salicylaldehyde hydrazine class AIE compound (1)
[0086] 4,4'-Dihydroxybenzophenone (10.0 g, 46.7 mmol) and urotropine (19.6 g, 140.1 mmol) were added to 240 g (about 160 mL) of trifluoroacetic acid. Stir in a heating reaction device at 80 degrees Celsius for 15 hours, and the system is in a clear and transparent solution state during the reaction. Turn off the heating, and after the temperature drops to room temperature, add 80 mL of water and stir at room temperature for 3 hours to obtain a turbid system with a large amount of precipitates. Utilize the mode of suction filtration to separate the solid crude product, and then utilize the method for column chromatography (100-200 mesh silica gel column, sherwood oil / dichloromethane is used as developing agent with the ratio of 4:1) to purify the solid crude product, Obtained N', N'-((1E,1'E)-(carbonylbis(6-hydroxyl-3,1-phenylene))bis(formimide))bis(benzohydrazide) 21.8g( Yield ab...
Embodiment 2
[0089] Embodiment 2: Synthetic preparation of salicylaldehyde hydrazine class AIE compound (2)
[0090]The synthesis method of the salicylaldehyde hydrazine type AIE compound (2) is the same as the method for synthesizing the above salicylaldehyde hydrazine type AIE compound (1) (Example 1). The starting material benzohydrazide was replaced by p-methoxybenzohydrazide (2.9 g, 17.7 mmol). (yield: 92%) Elemental analysis: C, 65.88; H, 4.51; N, 9.81. Mass spectrometry: 566.01.
[0091]
Embodiment 3
[0092] Embodiment 3: Synthetic preparation of salicylaldehyde hydrazine class AIE compound (3)
[0093] The synthesis method of the salicylaldehyde hydrazine type AIE compound (3) is the same as the method for synthesizing the above salicylaldehyde hydrazine type AIE compound (1) (Example 1). The starting material benzohydrazide was replaced by p-ethoxybenzohydrazide (3.2 g, 17.7 mmol). (yield: 92%) Elemental analysis: C, 66.82; H, 4.94; N, 9.39. Mass spectrometry: 594.62.
[0094]
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average thickness | aaaaa | aaaaa |
| The average thickness | aaaaa | aaaaa |
| The average thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com