Antibody inhibiting activated ras in cell by internalizing into cytosol of cell, and use thereof
The technology of an antibody, YISRTSHT-X21-X22-YADSVKG, is applied in the field of antibodies that inhibit activated Ras in cells through internalization into the cytoplasm of cells and its use. Direct penetration, poor efficiency and other issues, to achieve the effect of high binding capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0195] Example 1. Avi-KRas binding to GppNHp G12D protein preparation
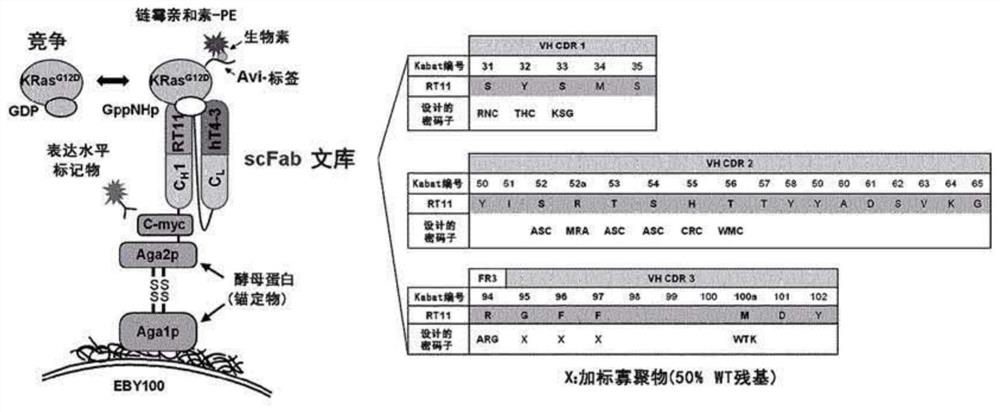
[0196] Build Avi-KRas G12D An antigen comprising an Avi tag (GLNDIFEAQKIEWHE) fused to its N-terminus for library selection. Build Avi-KRas G12D Antigens in order to minimize structural denaturation, which could cause problems in antigen biotinylation during library selection.
[0197] Specifically, KRas that does not contain the C-terminal hypervariable region was constructed by PCR G12DThe catalytic G domain of the protein (residues 1 to 169) was N-terminally fused with 8X his-tag and Avi-tag DNA using a GSG linker and using the restriction enzyme NcoI in the pET23 vector as a vector for E. coli expression and HindIII clones. Then, the constructed pET23-8Xhis-Avi-KRas was electroporated G12D (1-169) was transformed into E. coli strain BL21(DE3) plysE together with a vector (pBirAcm) encoding BirA as a biotin ligase for in vivo biotinylation, and then in the presence of 100 μg / ml ampicillin and 10 μ...
Embodiment 2
[0202] Example 2. Construction of RT11D-based Anti-Ras GTP iMab High Diversity Antibody Library and Selection of Heavy Chain Variable Region (VH) with Enhanced Ras GTP-specific Affinity
[0203] The anti-Ras·GTP iMab RT11 in the conventional patent (Korean Patent No. 10-1602876) highly specifically binds to Ras·GTP and exhibits biological activity in various Ras mutant cell lines, but exhibits about 12 nM This is a lower affinity than that of various antibodies in the IgG form. In addition, the anti-Ras GTP iMab RT11, which exhibits biological activity by inhibiting the binding between Ras GTP and effector molecules, can enhance the biological activity by improving the affinity with Ras GTP. Therefore, in addition to modifying (improving) the light chain variable region to confer tissue specificity to it in order to increase the therapeutic efficiency of anti-Ras·GTP iMab, the inventors attempted to increase the affinity of anti-Ras·GTP iMabRT11 for Ras·GTP.
[0204] The ligh...
Embodiment 3
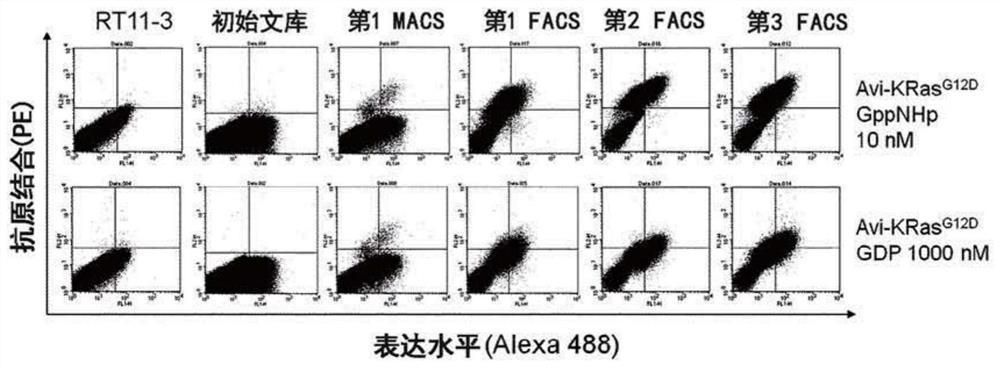
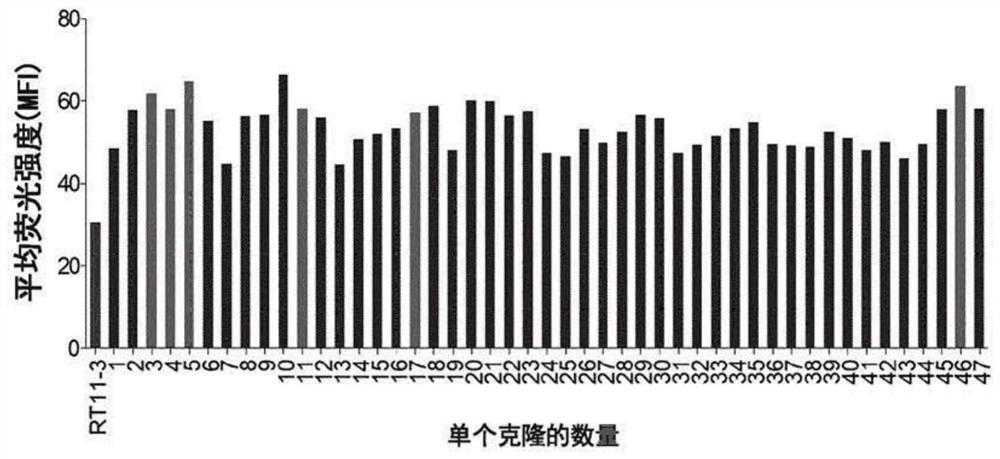
[0210] Example 3. KRas for GppNHp binding G12D Selection of heavy chain variable regions (VH) with improved affinity
[0211] Using GppNHp-bound KRas G12D The RT11-3-based affinity-improved library constructed in Example 2 was selected as the antigen.
[0212] Specifically, about 100 nM of purified GppNHp-bound Avi-KRas G12DReact for 1 hour at room temperature with yeast using SG-CAA medium (20g / L galactose, 6.7g / L yeast nitrogen base without amino acids, 5.4g / LNa 2 HPO 4 , 8.6g / L NaH 2 PO 4 , 5 g / L casamino acids) induced the expression of a single-chain Fab (scFab) type heavy chain variable region library on the cell surface. Then, the expression of Avi-KRas combined with GppNHp G12D Ligate the library to yeast at 4 °C with streptavidin Microbead TM (Miltenyi Biotec) reacted for 20 minutes, and then used MACS (Magnetic Activated Cell Sorting) to enrich and express Avi-KRas for GppNHp binding G12D Yeast with high affinity heavy chain variable regions. In the selecti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com