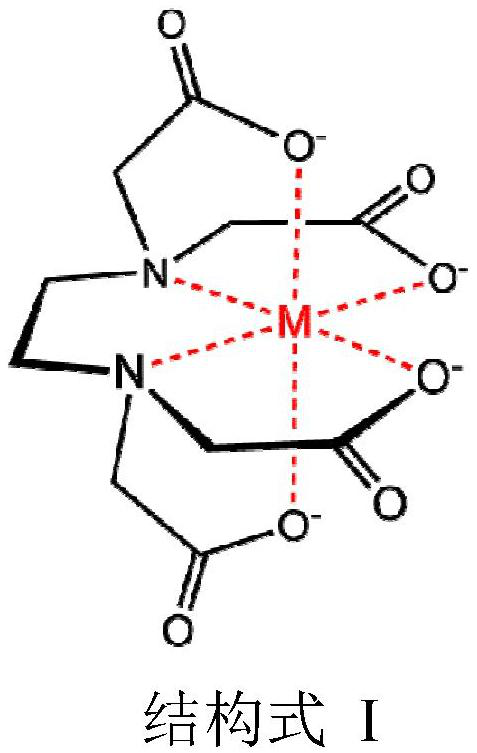
Ophthalmic preparation
An ophthalmic composition and weight technology, which is applied in the direction of medical preparations containing non-active ingredients, medical preparations containing active ingredients, organic active ingredients, etc., can solve problems that cannot be solved well, the cost of preparations becomes higher, and preparations Storage and use troubles and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment : Embodiment 1
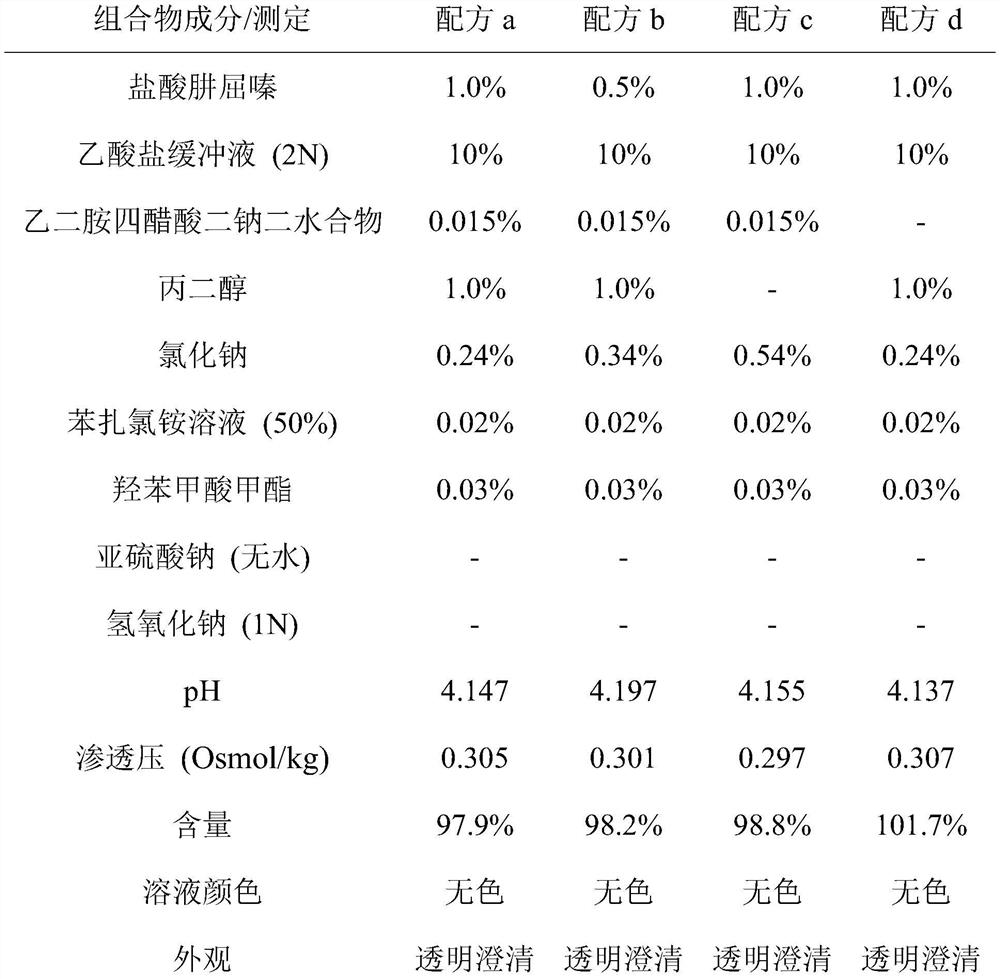
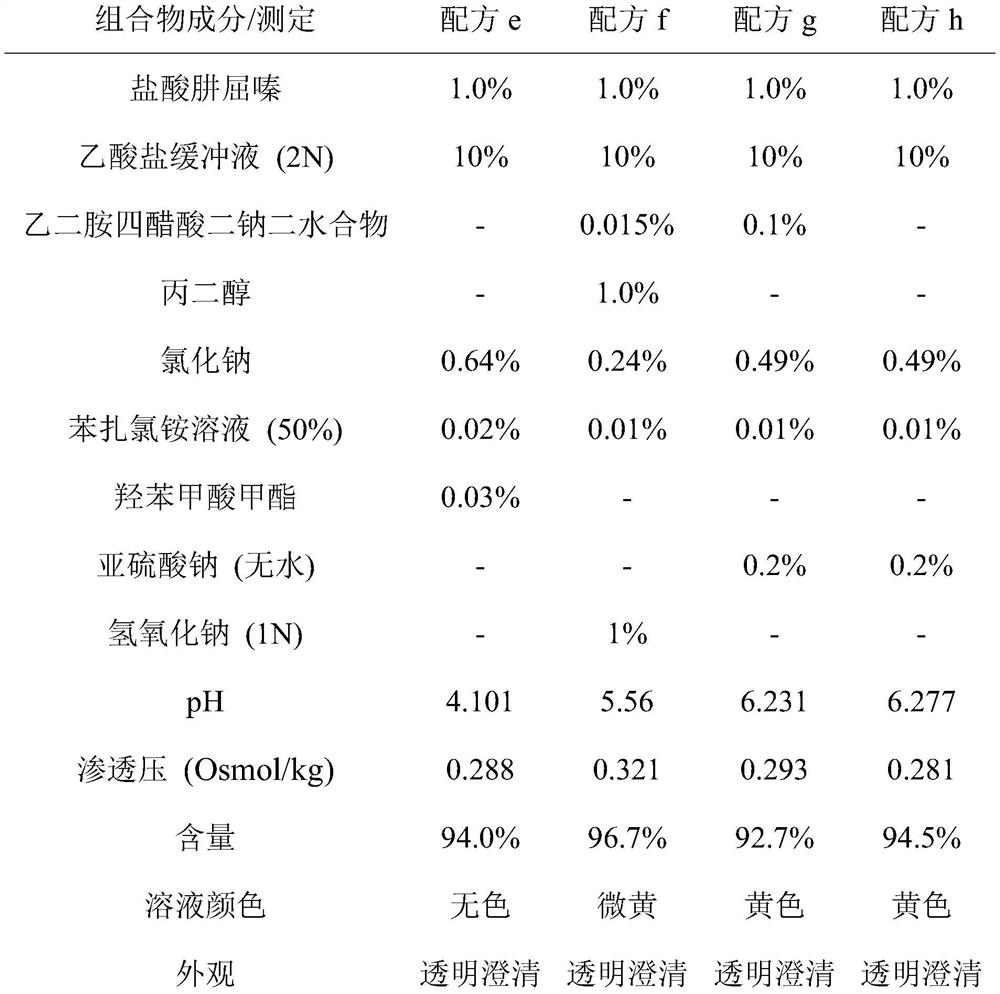
[0054] Examples: Example 1: Formulations can be prepared by stepwise addition of ingredients.
[0055] In an embodiment, a method for preparing an ophthalmic composition formulation comprises: first adding an appropriate amount of buffer solution to an appropriate amount of purified water under stirring. Purified water is preferably filled into containers of suitable size to accommodate the volume of the final formulation. Buffer solutions can be prepared separately or can be purchased commercially. In one non-limiting embodiment, the buffer solution is an acetate buffer solution. In one non-limiting embodiment, the acetate buffer solution comprises sodium acetate, acetic acid and purified water. In an exemplary embodiment, the pH of the acetate buffer solution is about 4.2 to 4.6. In a specific embodiment, the pH of the buffer is 4.2. As a non-limiting example, an acetate buffer solution can be prepared by adding 1.75 g of sodium acetate and 18.6 mL of acetic acid 2N into...
Embodiment 2
[0064] Example 2: The stability of the disclosed formulation was tested by accelerating the chemical or physical changes of the drug by increasing the storage temperature.
[0065] Eight hydralazine hydrochloride formulations of formulation a-formulation h in Example 1 were filled into glass sample vials. Formulations were stored at 40°C and 50°C to evaluate storage under accelerated chemical or physical changes of the drug. Measure the pH, osmolality (Osmol / kg) and content (%) of the solution active ingredient when the time is zero and one, two, three, four weeks, and observe the appearance and color of the solution.
[0066]The results are shown in Table 3 to Table 10, the initial solution of the hydralazine hydrochloride preparations of formula f, formula g and formula h has changed color at room temperature. After four weeks of storage at 40°C, formula a, formula b, formula c, formula d and formula e can all remain colorless, clear and transparent. After three weeks of s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com