Medicinal preparation containing hyaluronic acid, medicinal transdermal patch and preparation method of medicinal transdermal patch
A technology for pharmaceutical preparations and hyaluronic acid, which is applied in the field of medicine to achieve the effects of reducing irritation, improving bioavailability and reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1
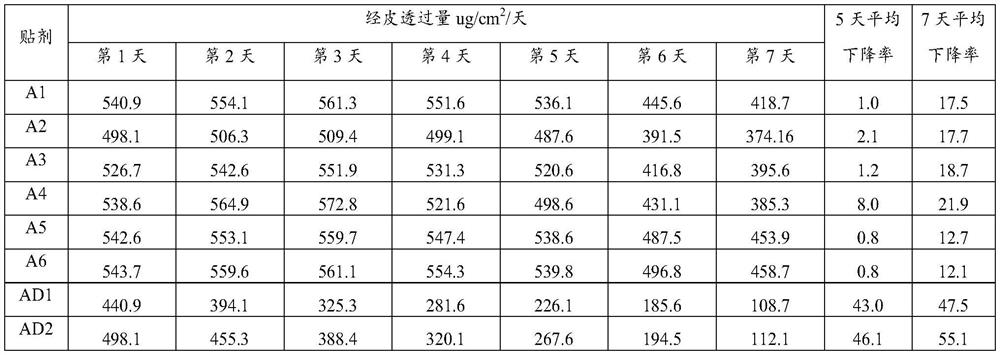
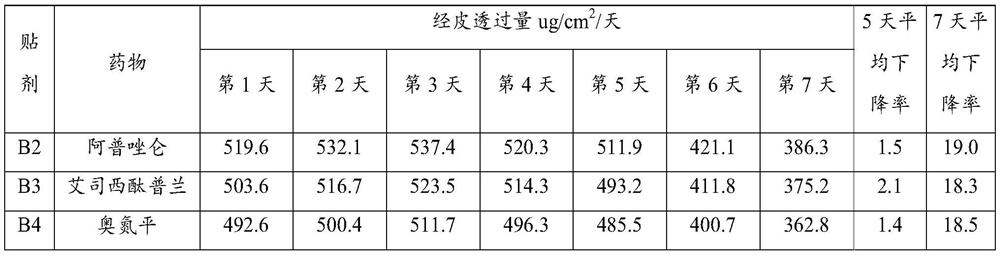
[0082] (1) Prepare packaged medicine
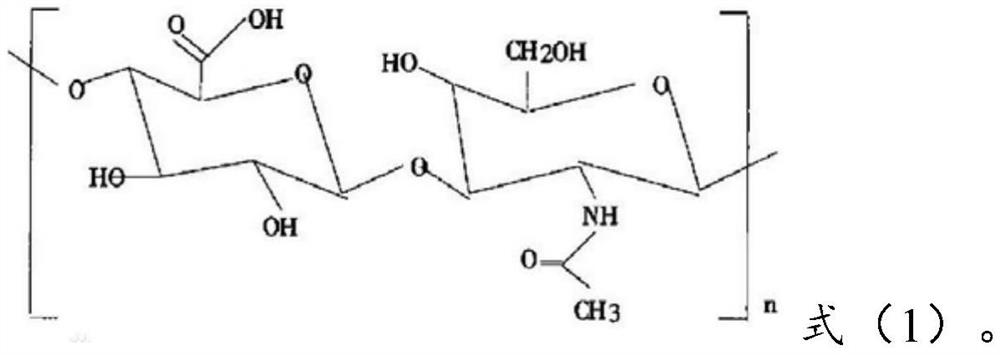
[0083] Dissolve 0.28g of lorazepam in an aqueous solution containing 0.42g of hyaluronic acid (molecular weight: 11300) represented by formula (1), and then carry out the process at an air inlet temperature of about 150°C and an outlet temperature of about 80°C. Spray-dry to obtain lorazepam hyaluronic acid coating with an average particle size of 268nm.
[0084] (2) Ingredients
[0085] Encapsulated drug: prepared in this embodiment;
[0086] Unpacked drug: Lorazepam, 0.1g;
[0087] Transdermal absorption enhancer: isopropyl myristate, 0.57g;
[0088] Drug-loaded matrix: 5.6g in total, of which 3.92g is 2852 pressure-sensitive adhesive (containing carboxyl group), 1.12g is 2287 pressure-sensitive adhesive (containing hydroxyl group), 1.12g is 4098 pressure-sensitive adhesive (no functional group) 0.56g;
[0089] Solvent: propylene glycol, 0.4 g.
[0090] (3) preparation of drug transdermal patch
[0091] Mix and dissolve the unwra...
Embodiment A2
[0094] (1) Prepare packaged medicine
[0095] Dissolve 0.25g of lorazepam in an aqueous solution containing 0.45g of hyaluronic acid (molecular weight: 7800) represented by formula (1), and then carry out the process at an inlet temperature of about 150°C and an outlet temperature of about 80°C. Spray-dry to obtain lorazepam hyaluronic acid coating with an average particle size of 396nm.
[0096] (2) Ingredients
[0097] Encapsulated drug: prepared in this embodiment;
[0098] Unpacked drug: Lorazepam, 0.1g;
[0099] Transdermal absorption enhancer: isopropyl myristate, 0.42g;
[0100] Drug-loading matrix: a total of 6g, of which 4.8g is 235A pressure-sensitive adhesive (containing carboxyl group), 0.6g is 2516 pressure-sensitive adhesive (containing hydroxyl group), 0.6g is 4098 pressure-sensitive adhesive (no functional group) 0.6g;
[0101] Solvent: butylene glycol, 0.3 g.
[0102] (3) preparation of drug transdermal patch
[0103] Mix and dissolve the unwrapped drug...
Embodiment A3
[0106] (1) Prepare packaged medicine
[0107] Dissolve 0.22g of lorazepam in an aqueous solution containing 0.26g of hyaluronic acid (molecular weight: 13,200) represented by formula (1), and then carry out the process at an inlet air temperature of about 150°C and an outlet air temperature of about 80°C. Spray-dry to obtain lorazepam hyaluronic acid coating with an average particle size of 314nm.
[0108] (2) Ingredients
[0109] Encapsulated drug: prepared in this embodiment;
[0110] Unpacked drug: Lorazepam, 0.1g;
[0111] Transdermal absorption enhancer: Span 20, 0.70g;
[0112] Drug-loaded matrix: 4.67g in total, of which 2.8g is 2852 pressure-sensitive adhesive (containing carboxyl group), 1.87g is 2516 pressure-sensitive adhesive (containing hydroxyl group);
[0113] Solvent: n-butanol, 0.5 g.
[0114] (3) preparation of drug transdermal patch
[0115] Mix and dissolve the unwrapped drug prepared in this example with a solvent, and then fully mix with the wrapped...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com