Production process of ethyl bromodifluoroacetate
A technology of ethyl difluorobromoacetate and production process, applied in the field of chemistry, can solve problems such as increasing production costs, and achieve the effects of cost saving, speed control, and utilization rate improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
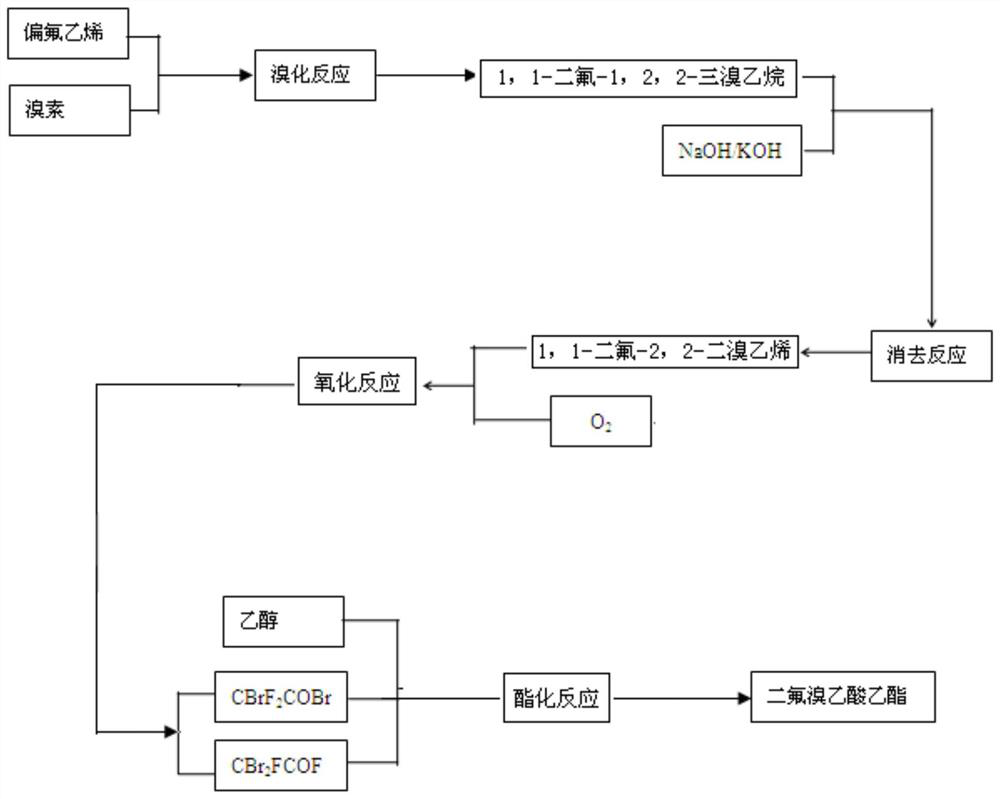
[0023] Such as figure 1 Shown, a kind of production technique of ethyl difluorobromoacetate comprises the steps:
[0024] Step 1. Bromination reaction: The bromine is metered and sucked into the bromination reaction kettle by vacuum, and vinylidene fluoride is used as a raw material to generate 1,1-difluoro-1,2 through light catalysis and bromine addition reaction, 2-Tribromoethane; the specific process is as follows: put vinylidene fluoride into a reaction bottle containing bromine, and add under light until the red color of the system fades to obtain 1,1-difluoro-1,2,2 - Tribromoethane, the content of 1,1-difluoro-1,2,2-tribromoethane is greater than 99%.
[0025] Step 2. Elimination reaction: Add the generated 1,1-difluoro-1,2,2-tribromoethane dropwise into a certain concentration of liquid caustic soda, and carry out the reaction of eliminating hydrogen bromide by raising the temperature to generate 1,1-difluoro -2,2-dibromoethylene; the specific process is as follows: W...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com