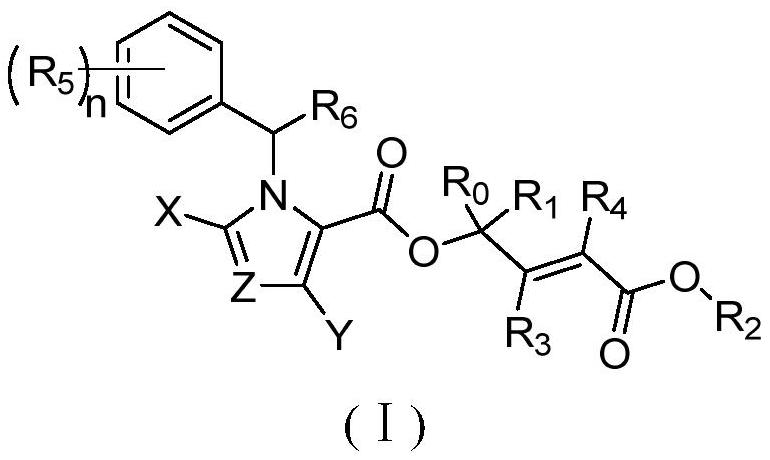
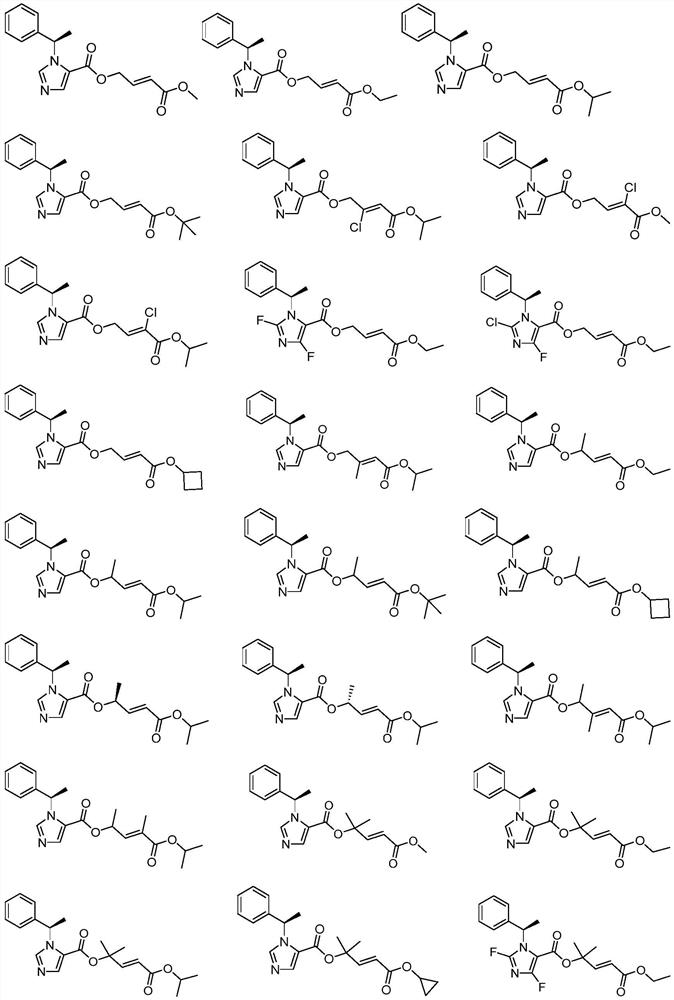
Nitrogen-containing heterocyclic compound as well as preparation method and application thereof
A nitrogen-heterocyclic compound and composition technology, applied in the field of nitrogen-containing heterocyclic compounds, can solve problems such as unpredictable recovery time, lack of effective coping methods for patients, and delayed wake-up time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] Dissolve 1 mmol of etomidate acid (CAS: 56649-48-0) in 20 mL of N,N-dimethylformamide (DMF), add 1 mmol of 4-bromo-crotonic acid methyl ester (CAS: 1117-71-1 ), add 2mmol anhydrous potassium carbonate powder. Stir at room temperature for 20 minutes and filter. The filtrate was washed into 120 mL of water, extracted with 50 mL of ethyl acetate, and the organic layer was washed once with water. Add an appropriate amount of anhydrous sodium sulfate to dry the organic layer for 8 hours, filter, evaporate the filtrate to dryness under reduced pressure, and purify the residue by silica gel column chromatography (cyclohexane v / ethyl acetate v=3:1) to obtain a colorless transparent oil 1.2 g, yield: 38.2%.
[0048] 1 HNMR (CDCl 3 ,400MHz)δ:1.85(3H,d,J=8Hz),3.82(3H,s),4.80~4.92(2H,m),5.99~6.04(1H,m),6.27~6.33(1H,m), 6.93~6.96(1H,m),7.16~7.32(m,5H),7.75(1H,s),7.86(1H,s).
Embodiment 2
[0050]
[0051] Dissolve 1mmol etomidate acid (CAS:56649-48-0) in 20mL N,N-dimethylformamide (DMF), add 1mmol ethyl 4-bromo-(E)crotonate (CAS:37746- 78-4), add 2 mmol of anhydrous potassium carbonate powder. Stir at room temperature for 20 minutes and filter. The filtrate was washed into 120 mL of water, extracted with 50 mL of ethyl acetate, and the organic layer was washed once with water. Add an appropriate amount of anhydrous sodium sulfate to dry the organic layer for 8 hours, filter, evaporate the filtrate to dryness under reduced pressure, and purify the residue by silica gel column chromatography (cyclohexane v / ethyl acetate v=3:1) to obtain a colorless transparent oil 1.61 g, yield: 49.1%.
[0052] 1 HNMR (CDCl 3 ,400MHz) δ: 1.30(3H,t,J=8Hz),1.87(3H,d,J=8Hz),4.21(2H,q,J=8Hz),4.81~4.93(2H,m),6.00~6.05 (1H,m),6.29~6.35(1H,m),6.95~6.99(1H,m),7.17~7.34(m,5H),7.77(1H,s),7.85(1H,s).
Embodiment 3
[0054]
[0055] Dissolve 1mmol etomidate acid (CAS:56649-48-0) in 20mL N,N-dimethylformamide (DMF), add 1mmol 4-bromo-isopropyl crotonate (CAS:29619-54- 3), add 2mmol anhydrous potassium carbonate powder. Stir at room temperature for 20 minutes and filter. The filtrate was washed into 120 mL of water, extracted with 50 mL of ethyl acetate, and the organic layer was washed once with water. Add an appropriate amount of anhydrous sodium sulfate to dry the organic layer for 8 hours, filter, evaporate the filtrate to dryness under reduced pressure, and purify the residue by silica gel column chromatography (cyclohexane v / ethyl acetate v=3:1) to obtain a colorless transparent oil 1.72 g, yield: 50.3%.
[0056] 1 HNMR (CDCl 3 ,400MHz) δ: 1.17(6H,d,J=8Hz), 1.85(3H,d,J=8Hz), 4.78~4.94(3H,m), 6.02~6.06(1H,m), 6.25~6.31(1H ,m), 6.92~6.96(1H,m), 7.18~7.36(m,5H), 7.71(1H,s), 7.83(1H,s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com