Preparation method of N-heterocyclic carbene catalytic axial chiral 1, 3-thiazine compound
An axial chirality, thiazide technology, applied in the direction of organic chemistry, organic chemistry, etc., to achieve the effect of good substrate universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
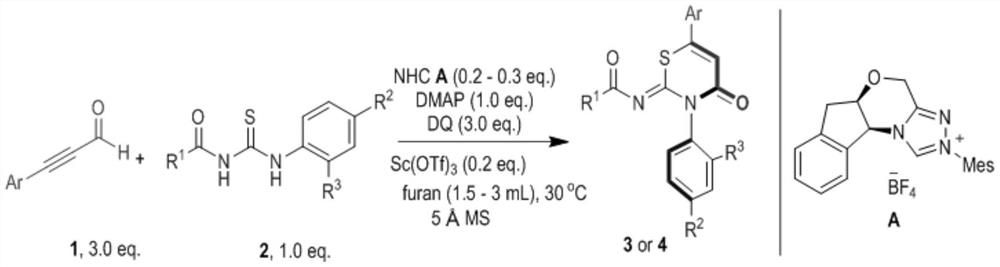
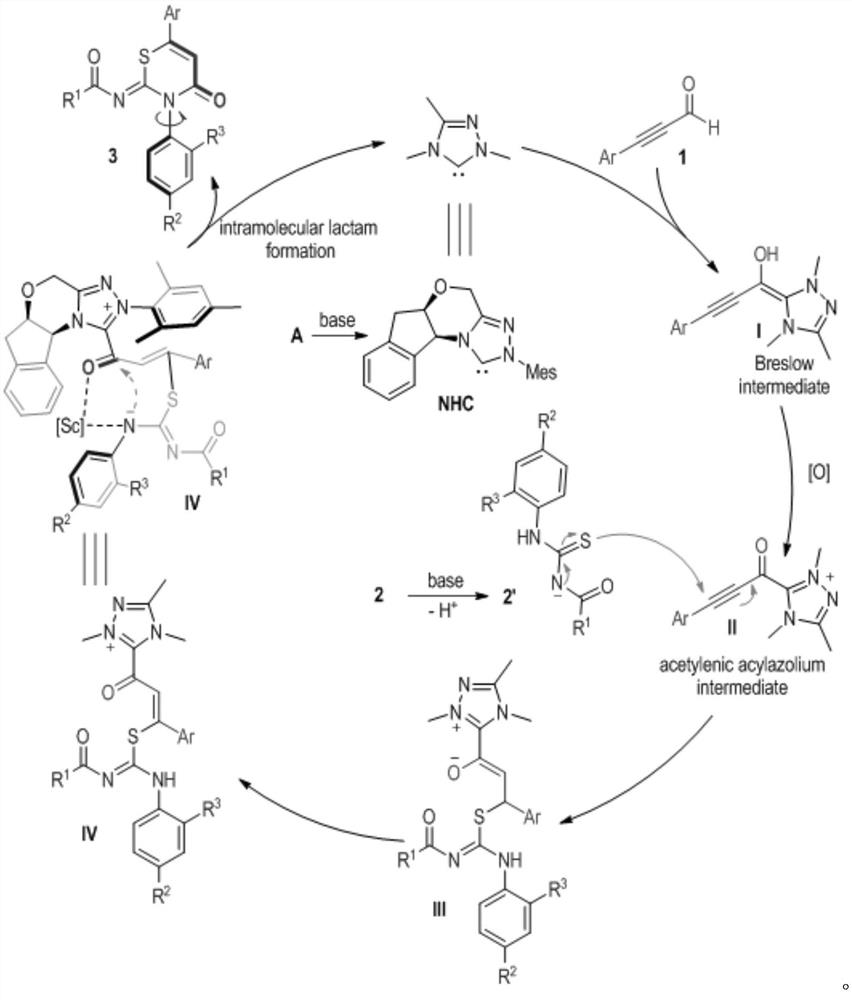
[0022] Synthetic route (I) for preparing (Z)-N-(4-keto-3-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene) substituted amide derivatives:
[0023]
[0024] Substituent Ar is Ph, R 1 for Ph, R 2 for H, R 3 For iPr, the preparation implementation method and conditions are as follows:
[0025] Weigh 0.1mmol (29.8mg) acylthiourea 2, 0.1mmol (12.2mg) 4-dimethylaminopyridine, 0.02mmol (8.4mg) nitrogen heterocyclic carbene catalyst A and 0.3mmol (122.4mg) oxidant DQ Added to a 4 mL reaction vial equipped with a magnetic stir bar, added 2 mL of furan, followed by 0.3 mmol (39.0 mg) of phenylpropynal 1. Cover the bottle cap and stir the reaction at 30°C for 12h. Wet sample loading, separation by column chromatography, eluent polar petroleum ether: ethyl acetate = 20:1 to obtain the target compound I 1 , Calculate the corresponding yield after weighing, and the compound is characterized by melting point instrument, polarimeter, nuclear magnetic resonance instrument NMR, high-resoluti...
preparation Embodiment 2
[0035] Substituent Ar is 4-CH 3 OC 6 h 4 , R 1 for Ph, R 2 for H, R 3 For iPr, preparation implementation method and conditions are the same as Preparation Example 1
[0036] (Z)-N-(3-(2-isopropylphenyl)-6-(4-methoxyphenyl)-4-one-3,4-dihydro-2H-1,3-thiazine -2-ylidene)benzamide (I 2 )
[0037]
[0038] White solid, yield: 64%, melting point: 177-178°C;
[0039] [α] 25 D =40.9 (c=0.5in CHCl 3 );
[0040] 1 H NMR (400MHz, CDCl 3 ), 7.29(t, J=7.7Hz, 2H), 7.13(d, J=7.8Hz, 1H), 7.01(d, J=8.8Hz, 2H), 6.88(s, 1H), 3.88(s, 3H), 2.85–2.75(m,1H),1.21 (d,J=6.8Hz,3H),1.14(d,J=6.8Hz,3H).
[0041] 13 C NMR (101MHz, CDCl 3 )δ174.4, 161.8, 161.5, 160.5, 149.7, 144.2, 135.1, 134.2, 131.9, 129.0, 128.2, 127.3, 127.2, 126.6, 125.9, 125.8, 125.5, 113.8, 113.0, 54.2, 28.6, 2
[0042] HRMS (ESI,m / z)calcd.forC 27 h 24 N 2 o 3 SH + :457.1580,found:457.1581.
[0043] HPLC analysis:97:3er(IA column, 25℃, hexane / iPrOH=80 / 20, 0.5mL / min, λ=254nm), Rt(minor)=27.2min, Rt(ma...
preparation Embodiment 3
[0045] Substituent Ar is 4-CH 3 C 6 h 4 , R 1 for Ph, R 2 for H, R 3 For iPr, preparation implementation method and conditions are the same as Preparation Example 1
[0046] (Z)-N-(3-(2-isopropylphenyl)-4-one-6-(p-methylphenyl)-3,4-dihydro-2H-1,3-thiazine-2 -ylidene)benzamide (I 3 )
[0047]
[0048] Yellow solid, yield: 63%, melting point: 148-150°C;
[0049] [α] 31 D =63.0 (c=0.5in CHCl 3 );
[0050] 1 HNMR (400MHz, CDCl 3 )δ7.75(dd, J=8.3,1.2Hz,2H),7.60(d,J=8.2Hz,2H),7.54–7.43(m,3H),7.40–7.35(m,1H),7.32–7.27 (m,4H),7.13(dd,J=7.8,0.9 Hz,1H),6.92(s,1H),2.85–2.75(m,1H),2.44(s,3H),1.21(d,J=6.9 Hz,3H),1.14 (d,J=6.8Hz,3H).
[0051] 13 C NMR (101MHz, CDCl 3 )δ175.5, 162.7, 161.6, 151.2, 145.2, 142.5, 136.1, 135.2, 132.9, 131.5, 130.1, 130.0, 129.3, 128.2, 127.6, 127.0, 126.8, 126.6, 115.5, 28.6, 23.9,
[0052] HRMS (ESI,m / z)calcd.for C 27 h 24 N 2 o 2 SH + :441.1637,found:441.1639.
[0053] UPLC analysis :98:2er(IA-U column, 25℃, hexane / iPrOH=90 / 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com