Method for detecting impurity bromoacetic acid in cefathiamidine
A cefathiamidine and detection method technology, applied in the detection field of bromoacetic acid, can solve the problems such as no reports on qualitative and quantitative detection, and achieve the effects of low detection limit, high sensitivity and good detection specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] First, prepare the following solutions:
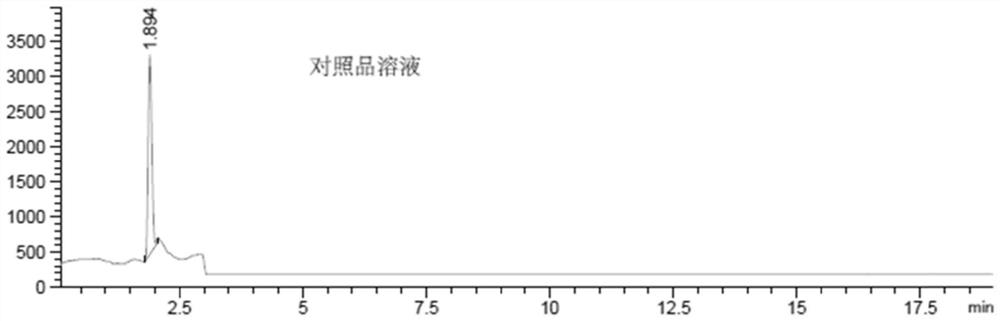
[0063] a) Prepare the reference substance solution:
[0064] Dissolve bromoacetic acid in water to prepare a bromoacetic acid solution with a concentration of about 250ng / ml as a reference solution;
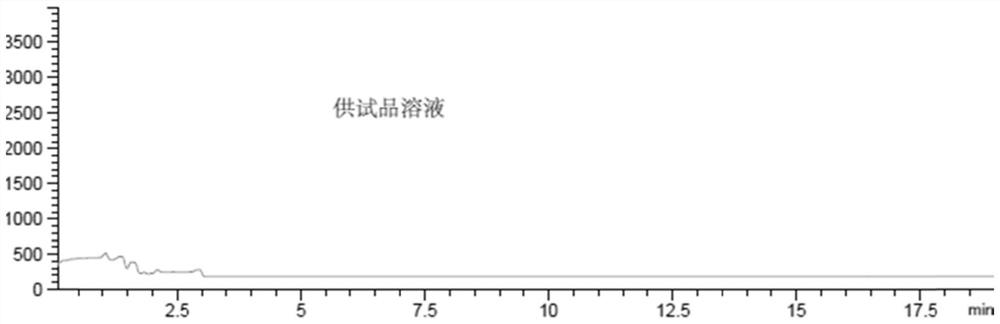
[0065] b) Prepare the test solution:
[0066] Dissolving cefathiamidine in water is prepared into a cefathiamidine solution with a concentration of about 100 mg / ml as the test solution;
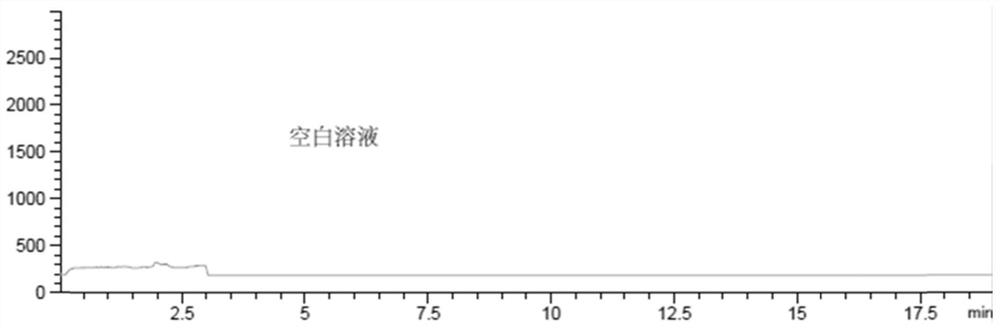
[0067] c) Blank solution: water
[0068] Then, according to the following liquid chromatography-mass spectrometry conditions, need testing solution, reference substance solution and blank solution are detected, and record collection of patterns:
[0069] Liquid chromatography-mass spectrometry conditions:
[0070] Instrument model Agilent 1260-Agilent 6120
[0071] Mass Spectrometry Conditions:
[0072] Scanning Mode: Selected Ion Monitoring (SIM)
[0073] Polarity: negative ion mode
[0074] Ion source: electrospray ion source
[0075] Mon...
Embodiment 2
[0091] Prepare the recovery solution for loading
[0092] Dissolve cefathiamidine and bromoacetic acid in water and prepare a solution containing about 100mg / ml cefathiamidine and 250ng / ml bromoacetic acid as a 100% sample recovery solution; in the same way, prepare bromoacetic acid content of 50% and 150% The solution is used as 50% and 150% sample recovery solution, wherein the concentration of bromoacetic acid is 125ng / ml and 375ng / ml respectively.
[0093] According to the liquid chromatography-mass spectrometry condition in embodiment 1, this 100% sample recovery solution is detected, and record collection of patterns, the result is as follows figure 2 shown.
[0094] It can be seen from the above that bromoacetic acid is completely separated from the main peak of the test product, the test product does not interfere with the detection of the target impurity peak, and the specificity of the method is good.
[0095] The recoveries of 50% sample recovery solution, 100% s...
Embodiment 3
[0097] Determining the limits of detection and quantitation
[0098] Accurately measure the reference substance solution (250ng / ml) in Example 1 and dilute step by step, until the solution whose peak height is 10 times of the baseline noise is the limit of quantification solution, and the solution whose peak height is 3 times of the baseline noise is the limit of detection solution.
[0099] According to the liquid chromatography-mass spectrometry condition of embodiment 1, limit of quantitation solution and limit of detection solution are detected, record collection of patterns, as attached Figure 3A and 3B As shown, the test results are as follows:
[0100]
[0101] From the above table, the signal-to-noise ratios of detection limit and quantification limit all meet the requirements. The quantitative limit of this method is about 12.95ng / ml (0.13ppm after conversion), which is less than the impurity limit requirement, so the sensitivity of this method meets the detec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com