Lyophilized pharmaceutical compositions for naked DNA gene therapy
A deoxyribonucleic acid, pharmaceutical technology, applied in the field of freeze-dried pharmaceutical compositions for naked deoxyribonucleic acid gene therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
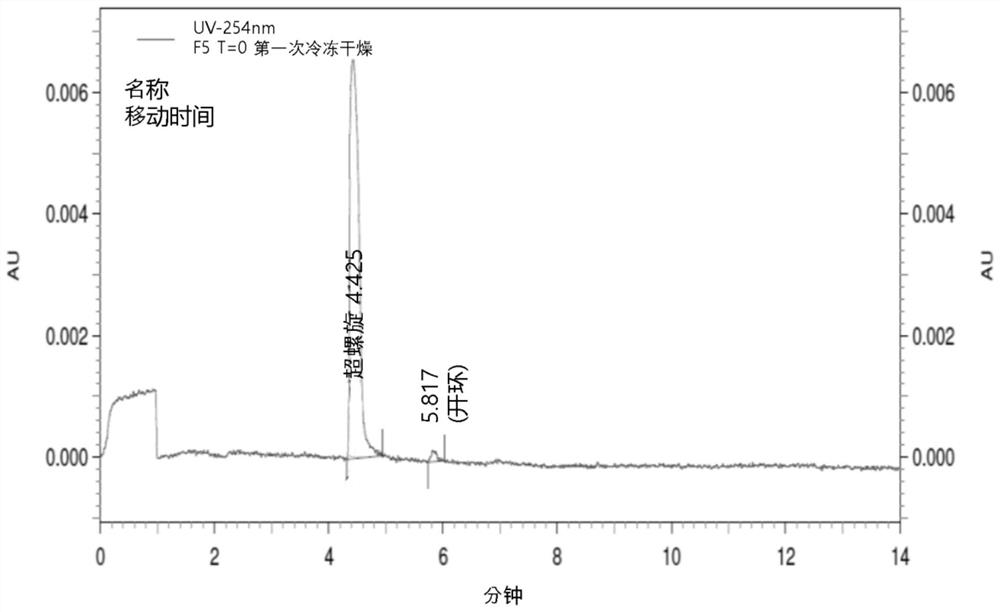
[0280] Example 1: Lyophilized composition of VM202 (Study 001)
[0281] 1.1 Pharmaceutical composition for trials comprising VM202
[0282] As shown in Table 1 below, various formulations containing VM202 were prepared. The above-mentioned VM202 plasmid as an active ingredient was obtained from frozen stock containing 1.6 mg / mL of VM202 in 0.9% sodium chloride or 1.3 mg / mL of VM202 in 0.9% sodium chloride.
[0283] Table 1
[0284]
[0285] *The control group (control) is the formulation described in US Pat. No. 8,389,492, the entire contents of which are incorporated herein by reference.
[0286] **KP8MS3N and 2MSN are lyophilized dosage forms of the same composition.
[0287] The above-mentioned dosage forms (formulation) are obtained by using materials and devices sold in the market, for example, potassium dihydrogen phosphate (Potassium Phosphate monobasic) (Spectrum, sample number PO200), dipotassium phosphate (PotassiumPhosphate dibasic) (EMD, sample number PX1570-...
Embodiment 2
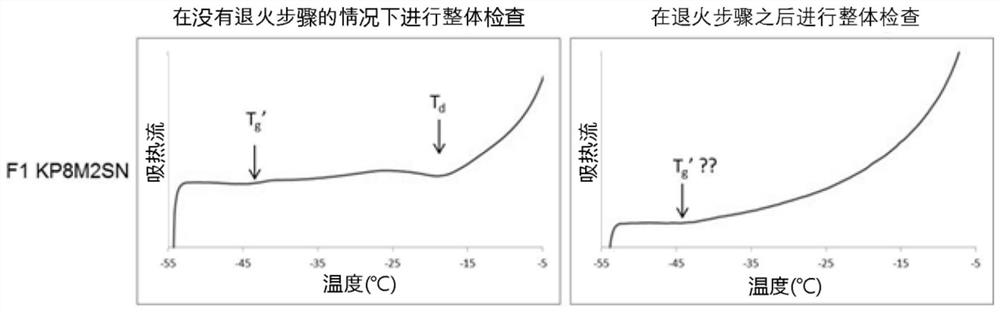
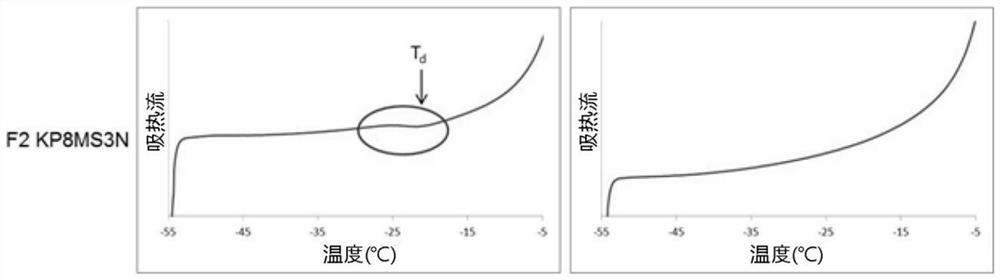
[0448] Example 2: Lyophilized composition of VM202 (Study 002)
[0449] To test the quality of VM202 in formulations with slight variations in pH and / or concentration of bulking agents and stabilizers, drug product formulations after lyophilization ), various lyophilized formulations containing VM202 were generated and analyzed.
[0450] 2.1 Experimental design
[0451] Material : The active pharmaceutical ingredient (API) examined in this study was VM202. The materials used in this study were structured as follows: The chemicals and materials used to formulate and analyze VM202 are as follows:
[0452] Table 26
[0453]
[0454]
[0455] Formulation parameter : In this study, the following parameters are fixed.
[0456] (1) Fill volume: 5mL
[0457] (2) API concentration: 0.5mg / mL
[0458] (3) Buffer concentration: 10mM potassium phosphate
[0459] (4) Sucrose concentration: 1%
[0460] In the above formulation, the following formulation parameters were exam...
Embodiment 3
[0547] Example 3. Freeze-dried composition of pTx-IGF-1X10
[0548] Among the lyophilized dosage forms, various lyophilized dosage forms containing pTx-IGF-1X10 were generated and analyzed for the quality of pTx-IGF-1X10. Next, the dosage forms listed in Table 37 were prepared for analysis.
[0549] Table 37
[0550] Dosage Form Code buffer pH expansion agent stabilizer PTX-IGF-1X10 concentration F1 10mM potassium phosphate 8 2% Mannitol 1.0% sucrose, 0.45% sodium chloride 0.5mg / ml F2 10mM potassium phosphate 7 2% Mannitol 1.0% sucrose, 0.45% sodium chloride 0.5mg / ml F3 10mM potassium phosphate 9 2% Mannitol 1.0% sucrose, 0.45% sodium chloride 0.5mg / ml F4 10mM potassium phosphate 8 1% Mannitol 1.0% sucrose, 0.45% sodium chloride 0.5mg / ml F5 10mM potassium phosphate 8 2% Mannitol 1.0% sucrose, 0.60% sodium chloride 0.5mg / ml F6 10mM potassium phosphate 8 2% Mannitol 1.0% sucrose, 0.90...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com