Quasi-three-dimensional phosphazene covalent organic framework material as well as preparation method and application thereof
A covalent organic framework, three-dimensional phosphazene technology, applied in separation methods, chemical instruments and methods, other chemical processes, etc., can solve the problems of high price, limited, insufficient three-dimensional COFs, etc., to improve pore permeability, improve Mass transfer rate and the effect of improving adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] A method for preparing a quasi-three-dimensional phosphazene covalent organic framework material, comprising the following steps:
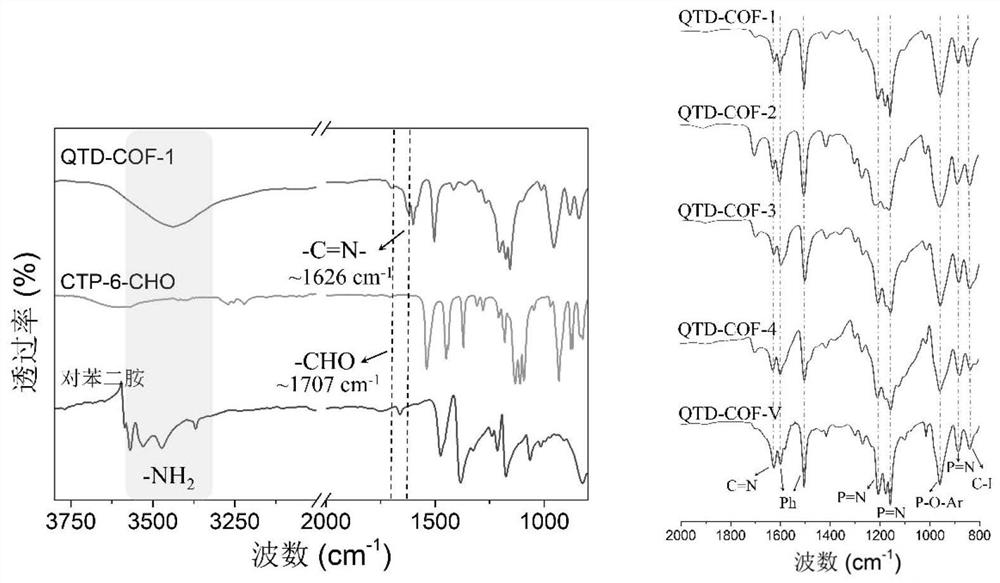
[0039] a. Dissolve p-hydroxybenzaldehyde in tetrahydrofuran, slowly add potassium carbonate to the system, stir at 0-5°C for 20-50 minutes, then slowly add dropwise the HCCP solution dissolved in tetrahydrofuran, ice-bath for 2 hours, and then stir at room temperature for 2- 4 days; the reaction was completed and filtered, the filtrate was removed from the solvent, and the obtained solid was extracted. After the organic phase was washed and dried, it was distilled under reduced pressure to obtain six (4-formylphenoxy)cyclotriphosphazene (CTP-6-CHO) ;
[0040] b. CTP-6-CHO and XH 4 Uniformly dispersed in the mixed solvent of o-dichlorobenzene and n-butanol, and then, the above-mentioned CTP-6-CHO dispersion system was added to the XH 4 In the dispersion system, mix evenly (or first add acetic acid solution and let stand at room temperature...
Embodiment 1 6
[0047] Embodiment 1 Preparation of six (4-formylphenoxy) cyclotriphosphazene (CTP-6-CHO)
[0048]
[0049] Dissolve 14.92g of p-hydroxybenzaldehyde in 300mL of THF (tetrahydrofuran), slowly add 33.4g of potassium carbonate to the system, stir in an ice bath for 30min, then slowly add 50mL of THF-dissolved HCCP (6.96g) solution dropwise, and ice-bath for 2h , Stir the reaction at room temperature for 3d. Filtrate after the reaction, distill the filtrate under reduced pressure, extract the obtained solid with dichloromethane, wash with saturated brine, dry the organic phase with anhydrous sodium sulfate, distill under reduced pressure to obtain a white solid, and recrystallize the solid powder in ethyl acetate , 14.98 g of white crystals were obtained with a yield of 87%.
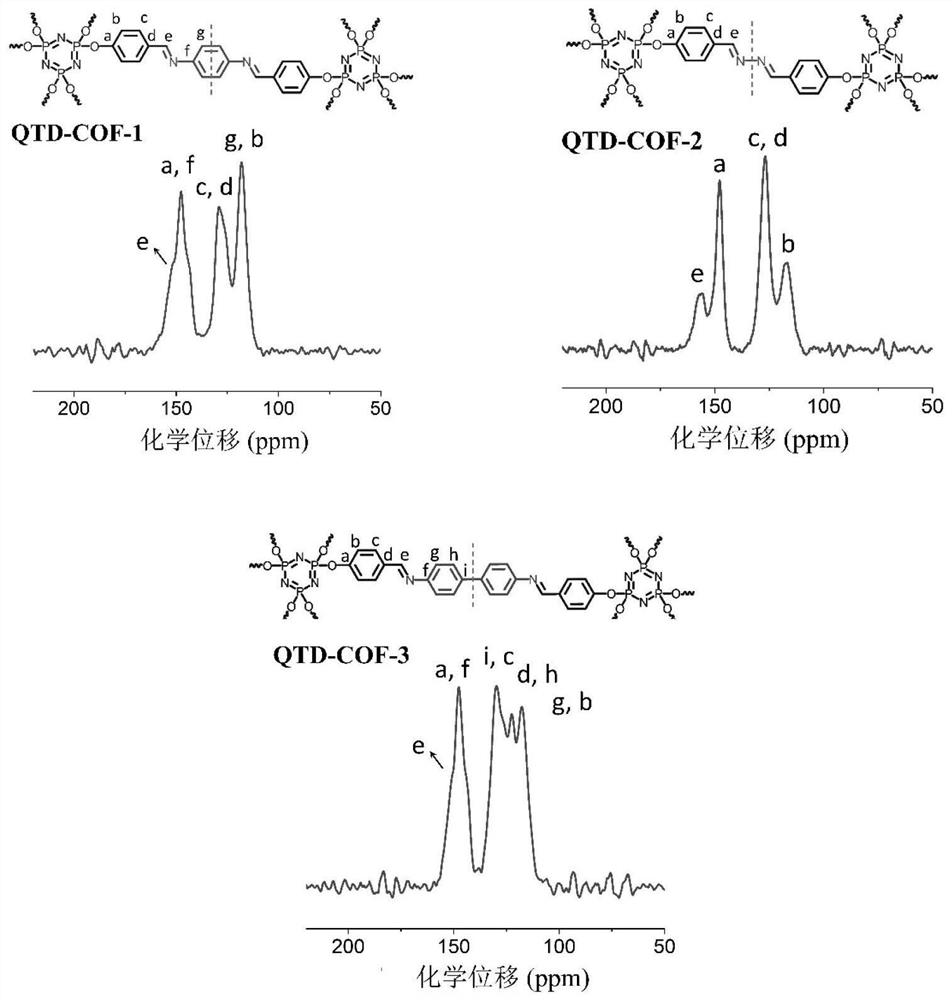
[0050] 1 H NMR (400MHz, DMSO) δ 9.92 (s, 6H), 7.79 (d, J = 8.6Hz, 12H), 7.18 (d, J = 8.5Hz, 12H).
[0051] 13 C NMR (101 MHz, DMSO) δ 192.17, 154.10, 134.08, 131.98, 121.57.
Embodiment 2
[0052] Example 2 Preparation of quasi-three-dimensional phosphazene covalent organic framework material QTD-COF-1
[0053]
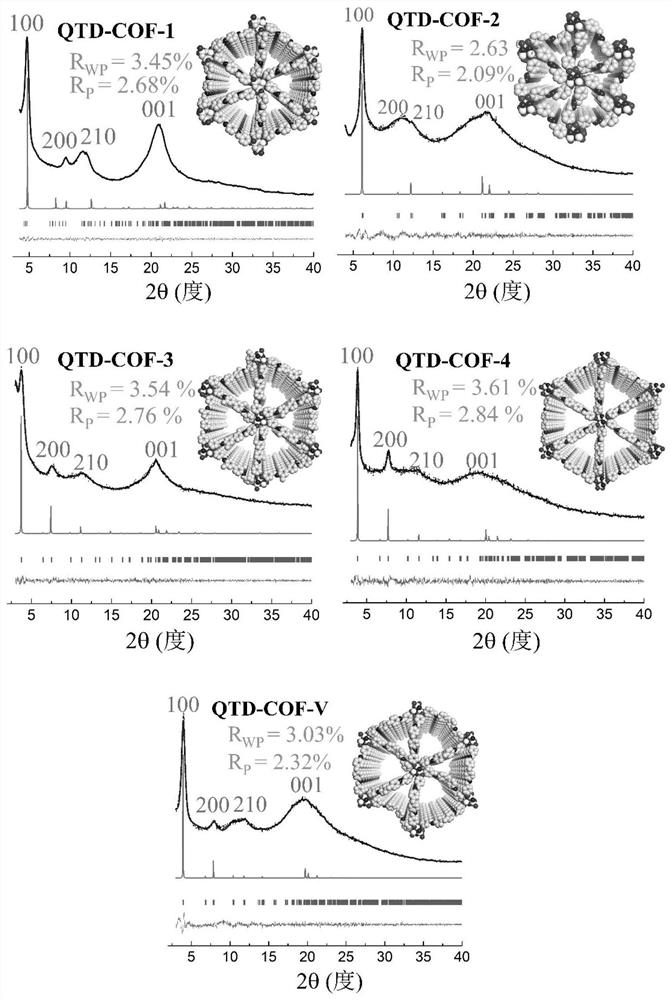
[0054] CTP-6-CHO (86mg) and p-phenylenediamine (33mg) were placed in a 15mL pressure-resistant bottle, and then o-dichlorobenzene and n-butanol (9:1v / v, 1.5mL) were added, and the two mixtures were ultrasonicated for 5 Minutes to obtain a uniform dispersion. Then, CTP-6-CHO was added to the p-phenylenediamine dispersion system, and the resulting suspension was briefly shaken for 10 s. Subsequently, acetic acid (6M, 0.3 mL) was slowly added, the bottle was sealed, and the reaction was left to stand at 120° C. for 7 days. The solid was collected by filtration and washed with DMF (N,N-dimethylformamide), acetone and THF respectively. The solid was vacuum-dried overnight at 50° C. to obtain a yellow crystalline solid with a yield of 91%, and the molecular formula was (C 20 h 14 N 3 o 2 P)n (% predicted / experimental: C 66.85 / 67.03, H 3.93 / 2.67, N 11....
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com