Synthetic method for carbonyl steroid-containing compound derivatization reagent
A technology of derivatization reagents and synthesis methods, applied in the direction of steroids, androstane derivatives, chemical instruments and methods, etc., can solve the problems of poor stability and low sensitivity of derivatized products, and achieve improved detection sensitivity, The effect of low cost and fast production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
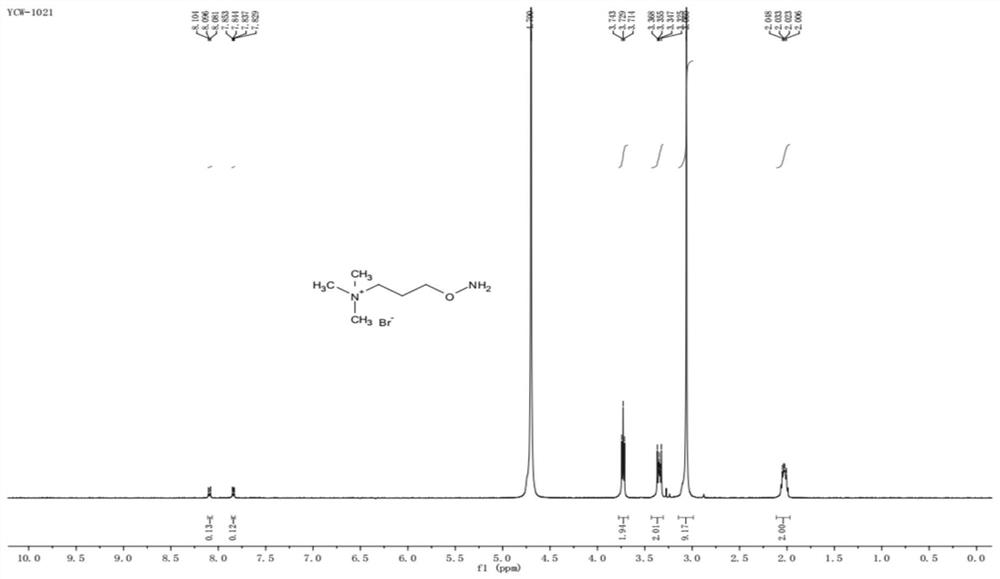
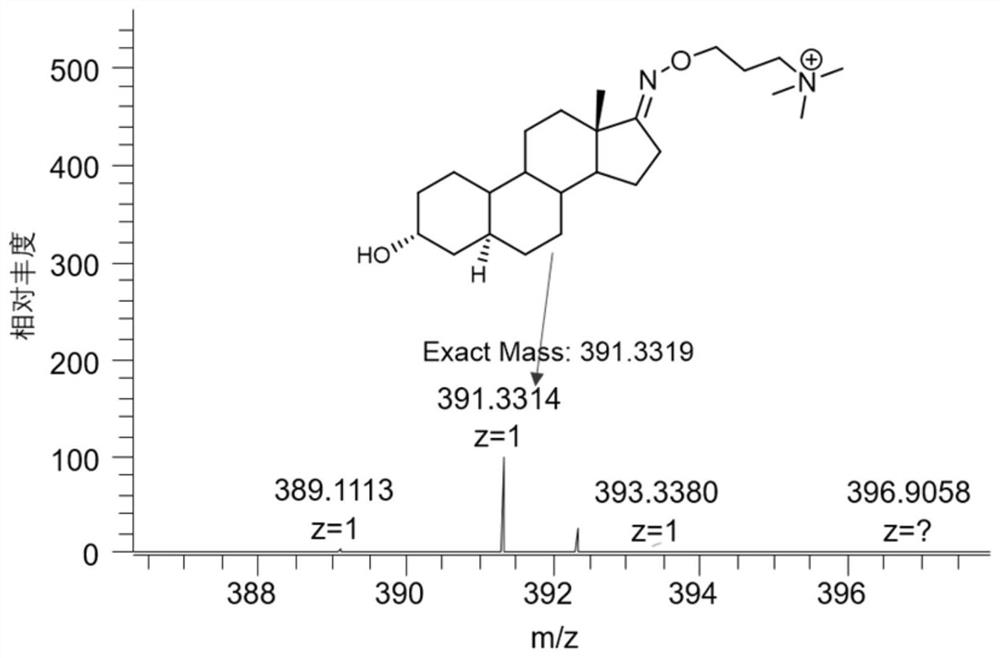
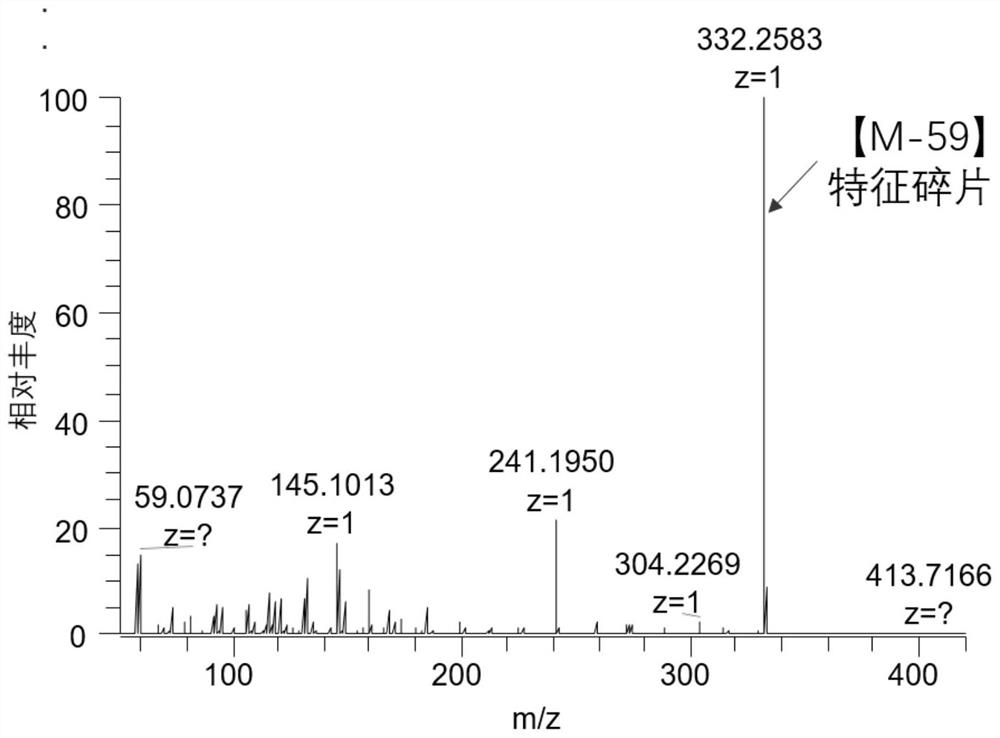
[0026] The synthesis method of the carbonyl-containing steroid compound derivatization reagent of the present invention. Add N-hydroxyphthalimide (2.01g, 12.3mmol), DMF (30mL), 1,3-dibromopropane (2.5mL, 24.5mmol) and triethylamine (3.3 mL, 23.7mmol), stirred at 25°C for 16h. Diluted with water and extracted with ethyl acetate, the organic phase was dried, filtered and then column chromatographed (petroleum ether / ethyl acetate=5 / 1) to obtain a white solid 2-(3-bromopropoxy)isoindoline- 1,3-diketone 2.3g, yield 66%. Add 2-(3-bromopropoxy)isoindoline-1,3-dione (2.3g, 8.10mmol) and trimethylamine tetrahydrofuran solution (24mL, 24mmol) into a 48mL sealed tube and stir at 40°C for 12h , trimethylamine is dissolved in tetrahydrofuran THF inside. The solid was collected by filtration and washed with dichloromethane to give a white solid 3-((1,3-dioxo-2-isoindolinyl)-oxyl)-N,N,N-trimethylpropyl Ammonium bromide 2.36g, the yield is 85%. Add 3-((1,3-dioxo-2-isoindolinyl)-oxyl)-N,N...
Embodiment 2
[0028]The synthesis method of the carbonyl-containing steroid compound derivatization reagent of the present invention. Add N-hydroxyphthalimide (2.01g, 12.3mmol), DMSO (30mL), 1,3-dibromopropane (2.5mL, 24.5mmol) and triethylamine (3.3 mL, 23.7mmol), stirred at 20°C for 16h. Diluted with water and extracted with ethyl acetate, the organic phase was dried, filtered and then column chromatographed (petroleum ether / ethyl acetate=5 / 1) to obtain a white solid 2-(3-bromopropoxy)isoindoline- 1,3-diketone 2.3g, yield 66%. Add 2-(3-bromopropoxy)isoindoline-1,3-dione (2.3g, 8.10mmol) and trimethylamine tetrahydrofuran solution (24mL, 24mmol) into a 48mL sealed tube and stir at 35°C for 10h . The solid was collected by filtration and washed with dichloromethane to give a white solid 3-((1,3-dioxo-2-isoindolinyl)-oxyl)-N,N,N-trimethylpropyl Ammonium bromide 2.36g, the yield is 85%. Add 3-((1,3-dioxo-2-isoindolinyl)-oxyl)-N,N,N-trimethylpropylammonium bromide ((2.36g , 6.88mmol), TH...
Embodiment 3
[0030] The synthesis method of the carbonyl-containing steroid compound derivatization reagent of the present invention. Add N-hydroxyphthalimide (2.01g, 12.3mmol), DMF (30mL), 1,3-dibromopropane (2.5mL, 24.5mmol) and triethylamine (3.3 mL, 23.7mmol), stirred at 30°C for 16h. Diluted with water and extracted with ethyl acetate, the organic phase was dried, filtered and then column chromatographed (petroleum ether / ethyl acetate=5 / 1) to obtain a white solid 2-(3-bromopropoxy)isoindoline- 1,3-diketone 2.3g, yield 66%. Add 2-(3-bromopropoxy)isoindoline-1,3-dione (2.3g, 8.10mmol) and trimethylamine tetrahydrofuran solution (24mL, 24mmol) into a 48mL sealed tube and stir at 50°C for 20h . The solid was collected by filtration and washed with dichloromethane to give a white solid 3-((1,3-dioxo-2-isoindolinyl)-oxyl)-N,N,N-trimethylpropyl Ammonium bromide 2.36g, the yield is 85%. Add 3-((1,3-dioxo-2-isoindolinyl)-oxyl)-N,N,N-trimethylpropylammonium bromide ((2.36g , 6.88mmol), Me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com