Crystal form of NUC-1031 single isomer and preparation method thereof
A technology of sp-1 and rp-1, which is applied in the field of compound Sp-1 in crystal form and its preparation, and can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] The preparation of embodiment 1 compound (61502):
[0083]
[0084] To a solution of 61501h (20g) in dichloromethane (60ml) was added 61501g (20.6g) at -80°C, followed by a solution of 19.3g triethylamine (diluted in 20ml dichloromethane). The mixture was stirred overnight at room temperature. To the mixture was added 61501f, followed by the addition of 19.3 g of triethylamine (diluted in 20 ml of dichloromethane) solution, and the mixture was stirred at room temperature for 4 h. The mixture was directly precipitated, and the residue was dissolved in ethyl acetate (200ml) and water (400ml). After separating the ethyl acetate, the aqueous phase was washed with ethyl acetate (2*100ml), the ethyl acetate phase was combined, and the acetic acid was washed with brine ethyl ether phase and dried over anhydrous sodium sulfate. Ethyl acetate was distilled off to obtain the target compound (61502), which was directly used in the next step of purification.
Embodiment 2
[0085] Preparation of Example 2 Compound (61501b):
[0086]
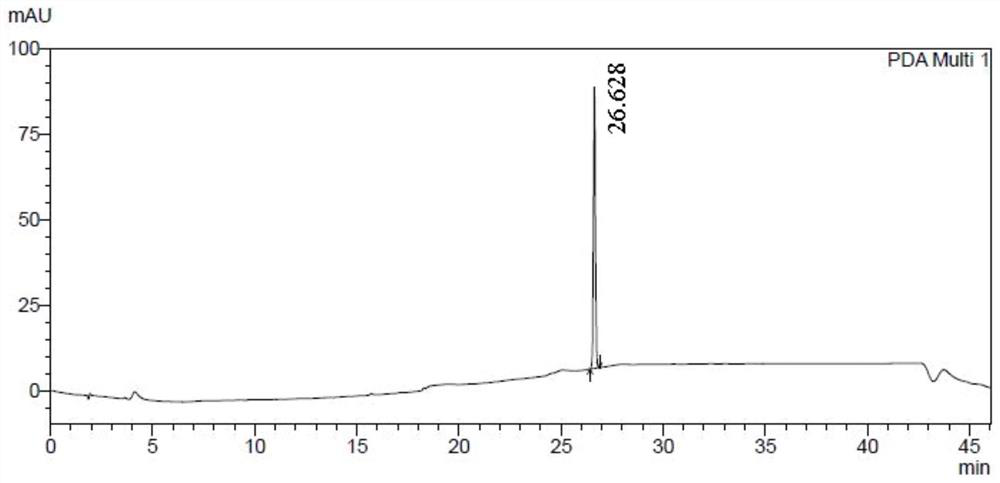
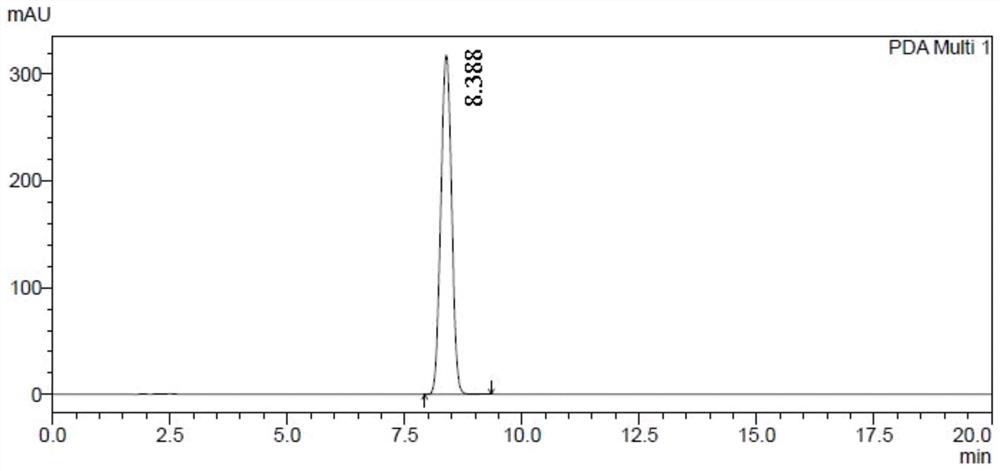
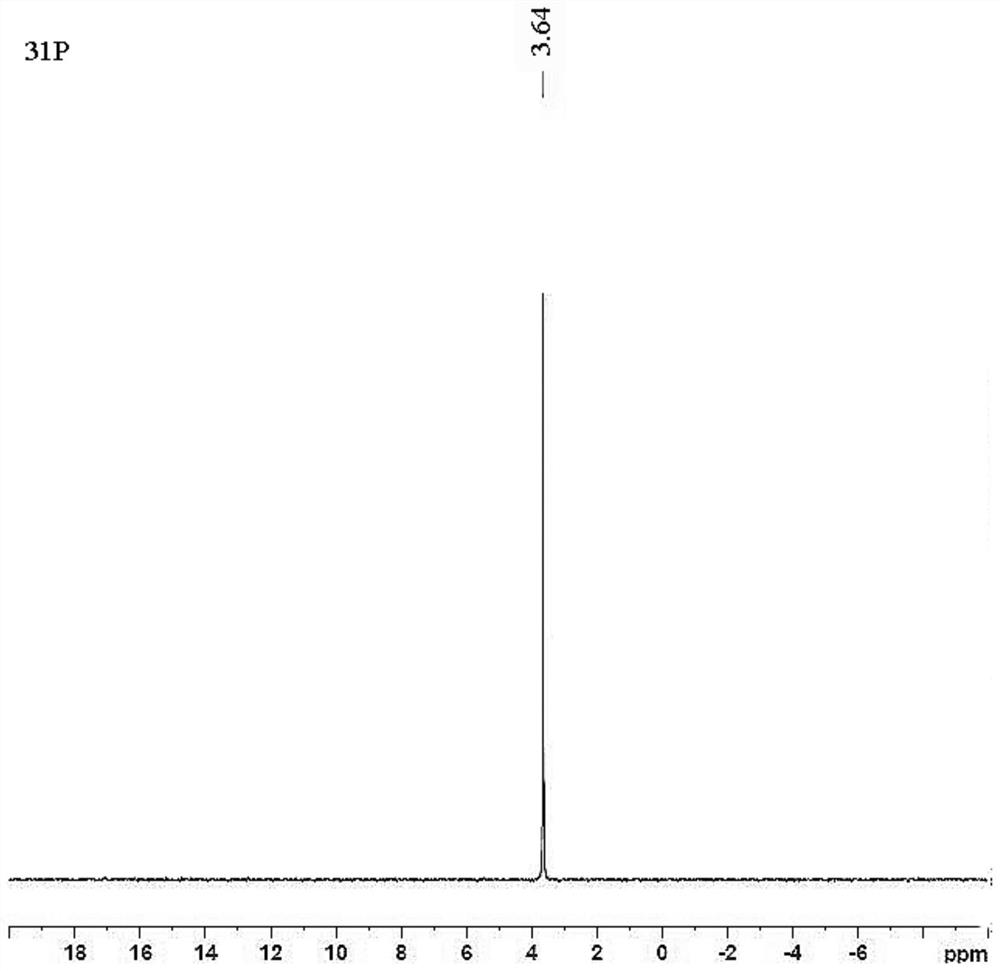
[0087] Compound 61502 (120g) was dissolved in ethyl acetate (240ml), stirred continuously, and petroleum ether (720ml) was slowly added dropwise at room temperature, crystals were precipitated, and the filtrate was removed by filtration to obtain compound 61501b (49.5g), with a yield of 41.2% , HPLC: 100.0% (as figure 1 shown).
Embodiment 3
[0088] The preparation of embodiment 3 compound (61501c):
[0089]
[0090] Add sodium carbonate (35.4g) to a mixed solution of compound (61501d) (20g) in tetrahydrofuran (200ml) and water (100ml) at room temperature, then add di-tert-butyl dicarbonate (17.5g), and stir at room temperature until the reaction is complete . The mixture was extracted with ethyl acetate (3*200ml), and the combined ethyl acetates were washed with brine and dried over anhydrous sodium sulfate. After distilling off the solvent, the residue was purified by silica gel chromatography (2.5%-10% methanol / dichloromethane) to obtain 18 g of compound (61501c), yield: 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com