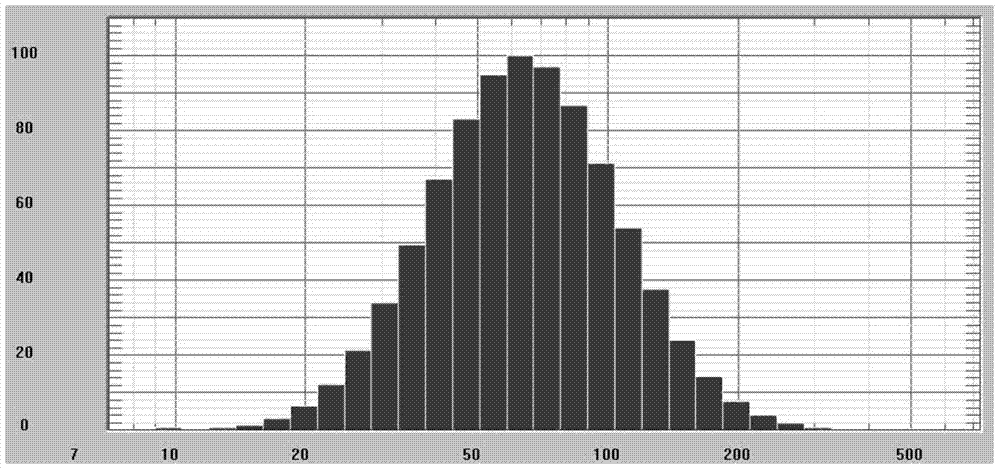
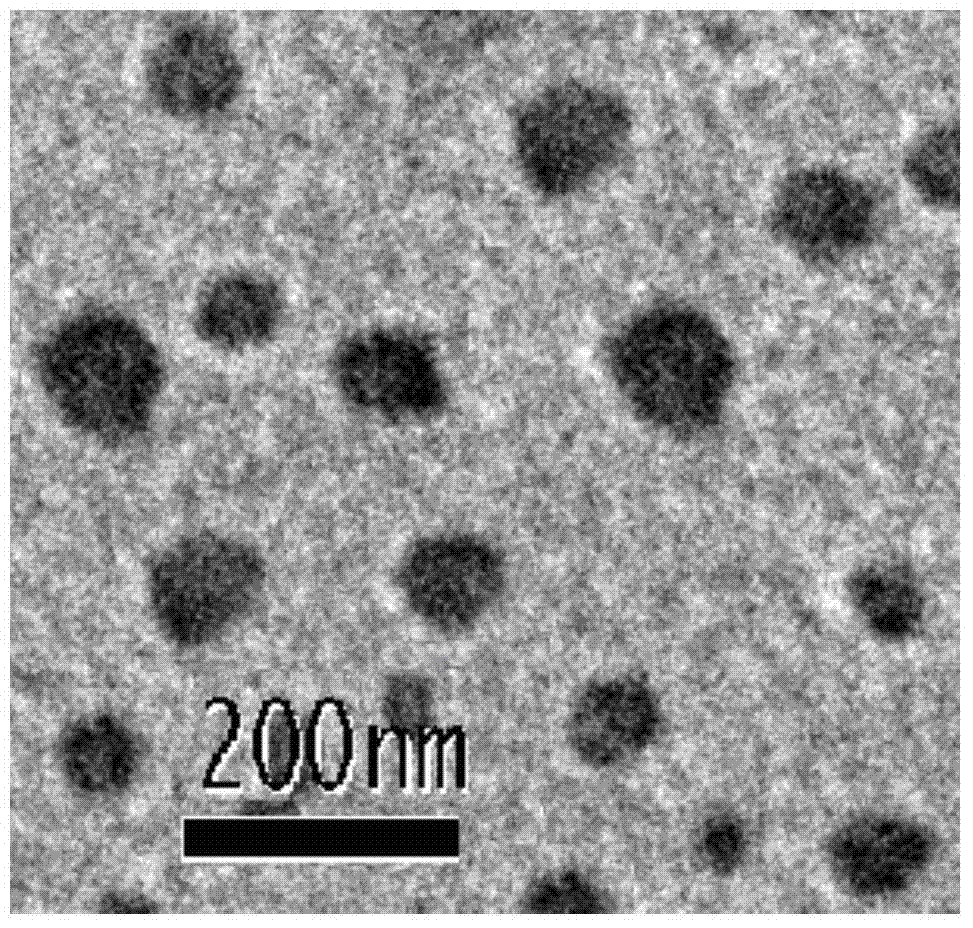
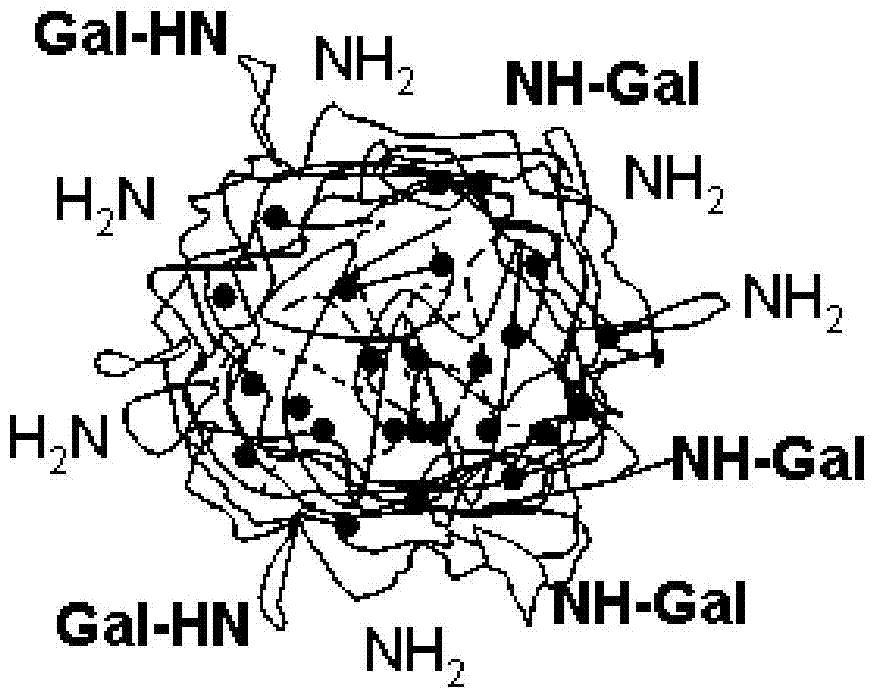
Nanoparticles, and preparation method, application and preparation thereof
A nanoparticle and organic solvent technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as difficulty in administration, impact on application and research, and low bioavailability , to improve the solubility and biocompatibility, and improve the relative bioavailability in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The preparation of embodiment 1 nanoparticle
[0057] Accurately weigh 80mg F68 and 50mg GC, dissolve in 20mL of 0.2% acetic acid aqueous solution as the water phase; accurately weigh 20mgα-Hed and 80mg phospholipids, dissolve in 4mL ethanol / acetone (1:1) mixed solvent as the organic phase, in Under the condition of magnetic stirring at 30°C, quickly inject the organic phase into the water phase, keep stirring for 5 minutes, then dilute to 70mL with distilled water, and cut it with a high-speed shearer at 20000rpm for 5min, and then homogenize it with a high-pressure homogenizer at 1000bar. After massaging for 5 minutes, it was filtered through a 0.45 μm microporous membrane to obtain a colloidal solution of nanoparticles, that is, α-hedera-N-lactosylchitosan nanoparticles (α-Hed-GC-NP) were obtained.
Embodiment 2
[0058] The preparation of embodiment 2 nanoparticles
[0059] Accurately weigh 80mg of F68 and 50mg of GC, dissolve in 20mL of 0.2% acetic acid aqueous solution as the water phase; accurately weigh 20mg of α-Hed and 80mg of phospholipids and dissolve in 4mL of ethanol as the organic phase. Inject the phase into the water phase quickly, keep stirring for 10min, then dilute to 70mL with distilled water, and cut it with a high-speed shearer at 20000rpm for 5min, then homogenize it at 1000bar for 5min with a high-pressure homogenizer, and pass through a 0.45μm microporous filter The nanoparticle colloidal solution was obtained by membrane filtration, that is, the α-hederin-N-lactosylchitosan nanoparticle (α-Hed-GC-NP) was obtained. The preparation of embodiment 3 nanoparticles
Embodiment 3
[0059] Accurately weigh 80mg of F68 and 50mg of GC, dissolve in 20mL of 0.2% acetic acid aqueous solution as the water phase; accurately weigh 20mg of α-Hed and 80mg of phospholipids and dissolve in 4mL of ethanol as the organic phase. Inject the phase into the water phase quickly, keep stirring for 10min, then dilute to 70mL with distilled water, and cut it with a high-speed shearer at 20000rpm for 5min, then homogenize it at 1000bar for 5min with a high-pressure homogenizer, and pass through a 0.45μm microporous filter The nanoparticle colloidal solution was obtained by membrane filtration, that is, the α-hederin-N-lactosylchitosan nanoparticle (α-Hed-GC-NP) was obtained. The preparation of embodiment 3 nanoparticles
[0060] Accurately weigh 80mg of F68 and 50mg of GC, dissolve in 20mL of 0.2% acetic acid aqueous solution as the aqueous phase; accurately weigh 20mg of α-Hed and 80mg of phospholipids and dissolve in 1mL of acetone as the organic phase. Inject the phase into...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com