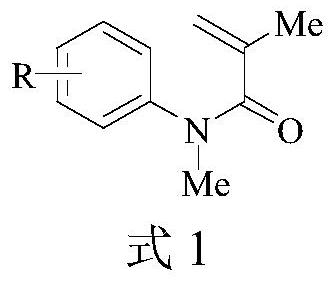
A kind of synthetic method of 1,3-dimethyl-3-hydroxymethylindolin-2-one compound
A technology for hydroxymethyl indoline and ketone compounds, which is applied in one field to achieve the effects of mild reaction conditions, excellent application value and wide selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
[0039] The following examples 1 to 4 are all reacted according to the following reaction equations, mainly to investigate the yield situation of different substrates reacting under optimal conditions:
[0040]
[0041] The specific operation steps are as follows: in a 10 mL round-bottomed flask, N-methyl-N-arylacrylamide (0.6 mmol), potassium monopersulfate (0.9 mmol) and acetonitrile (3 mL) were added in sequence. The obtained mixed solution was stirred and reacted at 90° C., and the reaction progress was tracked by thin layer chromatography, and the reaction time was 24 hours. After the reaction, the extract was concentrated in a rotary evaporator, and purified by column chromatography on silica gel using petroleum ether / ethyl acetate as the eluent.
Embodiment 1
[0043] Compound 1, 78% yield, 1,3-dimethyl-3-hydroxymethylindolin-2-one;
[0044] 3-(hydroxymethyl)-1,3-dimethylindolin-2-one;
[0045]
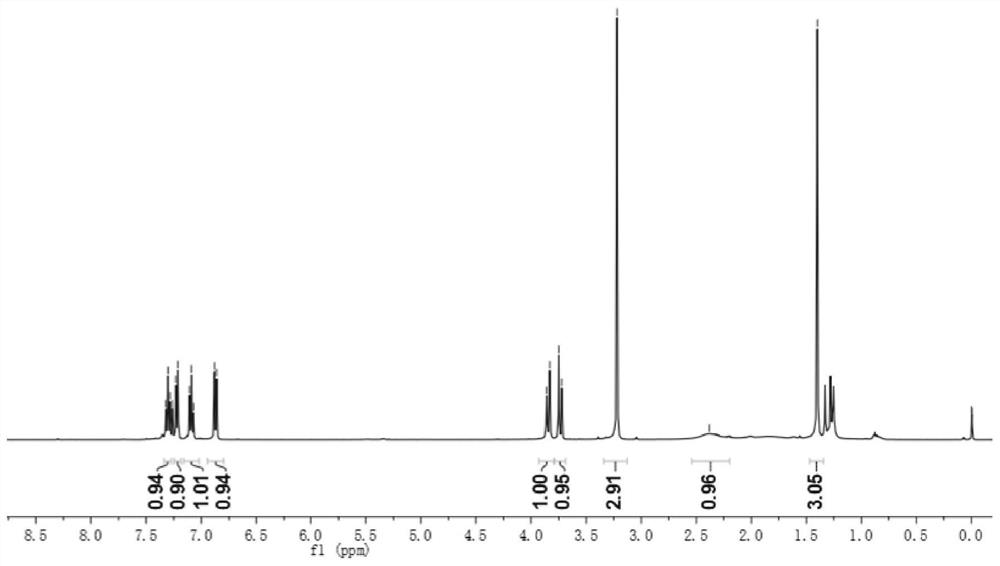
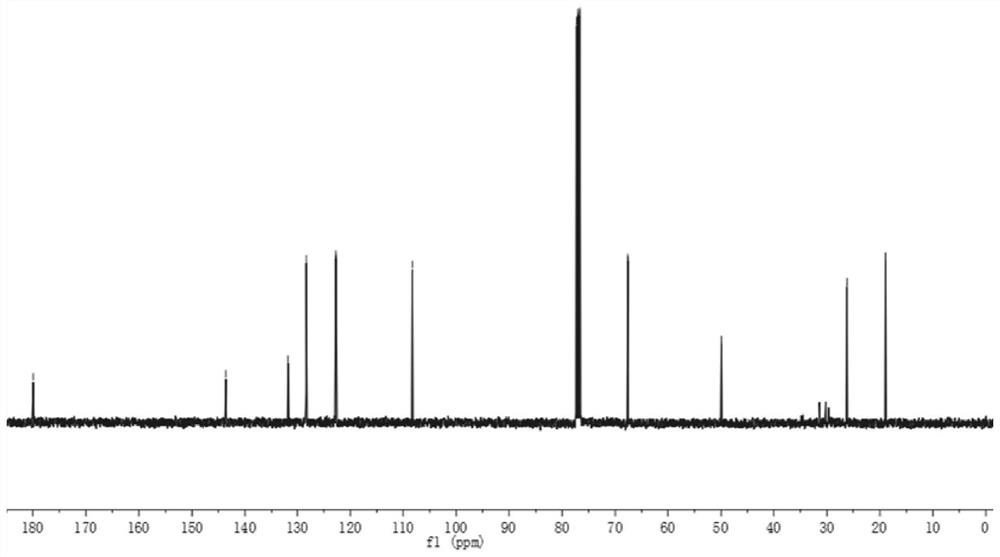
[0046] 1 H NMR (400MHz, CDCl 3 ,ppm)δ7.30(t,J=7.7Hz,1H),7.22(d,J=7.3Hz,1H),7.09(t,J=7.5Hz,1H),6.87(d,J=7.8Hz, 1H), 3.85(d, J=10.8Hz, 1H), 3.74(d, J=10.8Hz, 1H), 3.22(s, 3H), 2.38(s, 1H), 1.40(s, 3H);
[0047] 13 C NMR (100MHz, CDCl 3 ,ppm)δ180.0,143.6,131.8,128.3,122.7,122.7,108.3,67.6,49.9,26.2,19.0;
[0048] HRMS(ESI)m / z Calcd for C 11 H 13 NO 2 + [M + ]: 191.0946; found: 191.0948.
Embodiment 2
[0050] Compound 2, 82% yield, 3-(hydroxymethyl)-1,3,5-trimethylindolin-2-one;
[0051]
[0052] 1 H NMR (400MHz, CDCl 3 ,ppm)δ7.10(d,J=7.8Hz,1H),7.03(s,1H),6.77(d,J=8.0Hz,1H),3.84(d,J=10.8Hz,1H),3.72( d, J=10.8Hz, 1H), 3.21(s, 3H), 2.35(s, 3H), 1.40(s, 3H);
[0053] 13 C NMR (100MHz, CDCl 3 ,ppm)δ179.9,141.2,132.4,131.7,128.6,123.6,108.1,67.7,49.9,26.2,21.1,19.0;
[0054] HRMS(ESI)m / z Calcd for C 12 H 15 NO 2 + [M + ]: 205.1103; found: 205.1107.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com