Hybridoma cell line 3g7 1B10, anti-GII.4 type norovirus P protein monoclonal antibody and application
A technology of 3G71B10 and hybridoma cell lines, applied in the direction of antiviral immunoglobulin, immunoglobulin, chemical instruments and methods, etc., can solve the problems of missed detection, lack of cross-reactivity, and inability to satisfy human norovirus, etc. Achieve good cross-reactivity, good pairing effect, and clear results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of hybridoma cell lines and acquisition of monoclonal antibodies
[0025] 1. Preparation of GII.4 Norovirus P protein immunogen
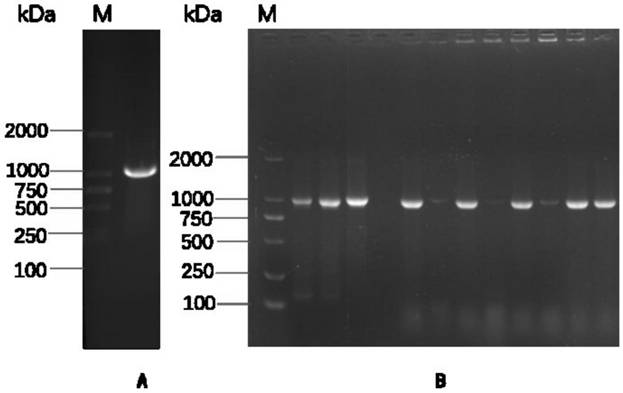
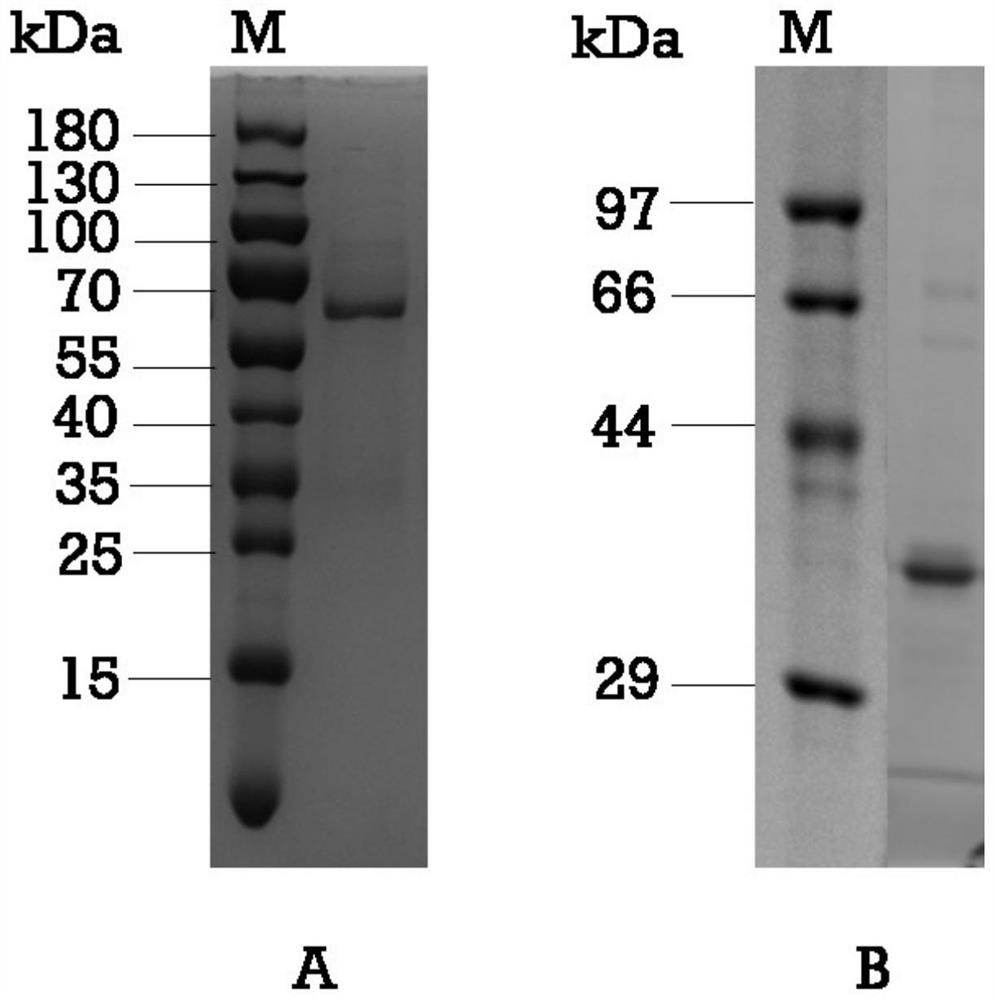
[0026] The amplification primers were designed according to the P region of GII.4-2012 type GZ2014-L307 strain (GenBank KT202798), the upstream primer P1 and the downstream primer P2 respectively introduced BamHI and EcoRI restriction sites (underlined part), and at the 5' of the downstream primer The addition of the CDCRGDCFC amino acid sequence (part in italics) at the end can promote the formation of Norovirus P particles as shown in Table 1. PCR amplification was performed with GII.4-2012 type GZ2014-L307 strain cDNA as a template, and the amplified product and pGEX-4T-1 vector (GE General Medical, USA) were subjected to BamHI and EcoRI double digestion respectively. The amplified product was ligated into the pGEX-4T-1 vector, transformed into E. coli BL21 competent cells, and verified by colony PCR and sequencing a...
Embodiment 2
[0041] Example 2 Establishment of double-antibody sandwich ELISA method
[0042] 1. Screening of paired antibodies and determination of optimal working concentration
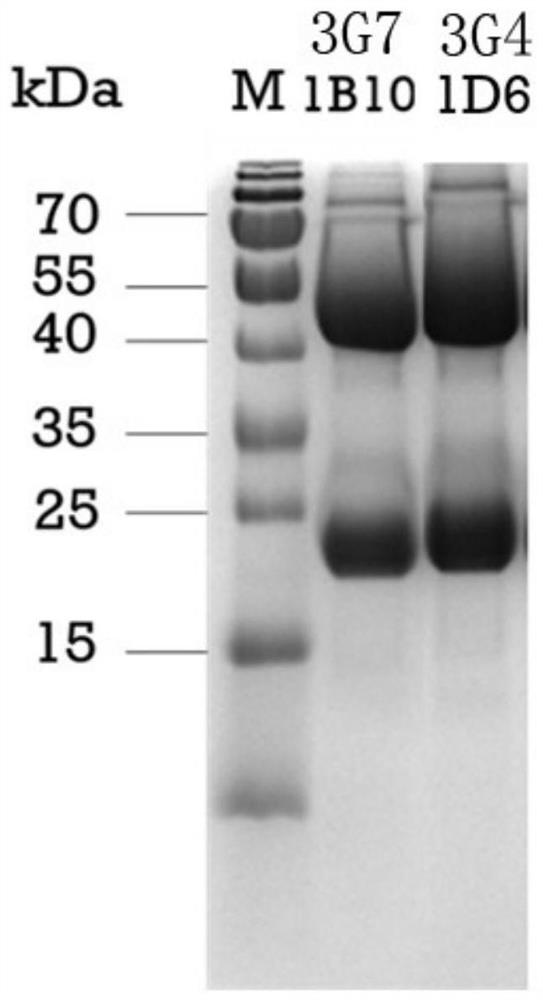
[0043] First, the two monoclonal antibodies were labeled according to the instructions of the horseradish peroxidase (HRP) conjugated labeling kit, and the sensitivity of the labeled antibody was determined by direct ELISA, and the detection antibody was determined. Using the double-antibody sandwich ELISA method, 1 μg / mL GII.4 P particles were used as the detection antigen, and the determined HRP-labeled monoclonal antibodies (HRP-3G7 1B10, HRP-3G4 1D6) were used as the detection antibodies, and the other two monoclonal antibodies ( 3G7 1B10, 3G4 1D6) were used as capture antibodies to pair with them, respectively, to determine the optimal paired antibody and the optimal working concentration. The results showed that the HRP-labeled 3G7 1B10 antibody (HRP-3G7 1B10) had the best sensitivity, so HRP-3G7 1B10 was...
Embodiment 3
[0046] Performance testing of the method of Example 3
[0047] The double-antibody sandwich ELISA method established in Example 2 is tested for performance, as follows:
[0048] 1. Specificity test
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com