A screening method for trace antibodies in the supernatant stage of hybridoma cells
A hybridoma cell and screening method technology, which is applied in the field of screening trace antibodies in the supernatant stage of hybridoma cells, can solve the problems of serum effect difference, blind preparation, etc., achieve shortened preparation time, high cost, and avoid expanded culture and protein production. The effect of the purification work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
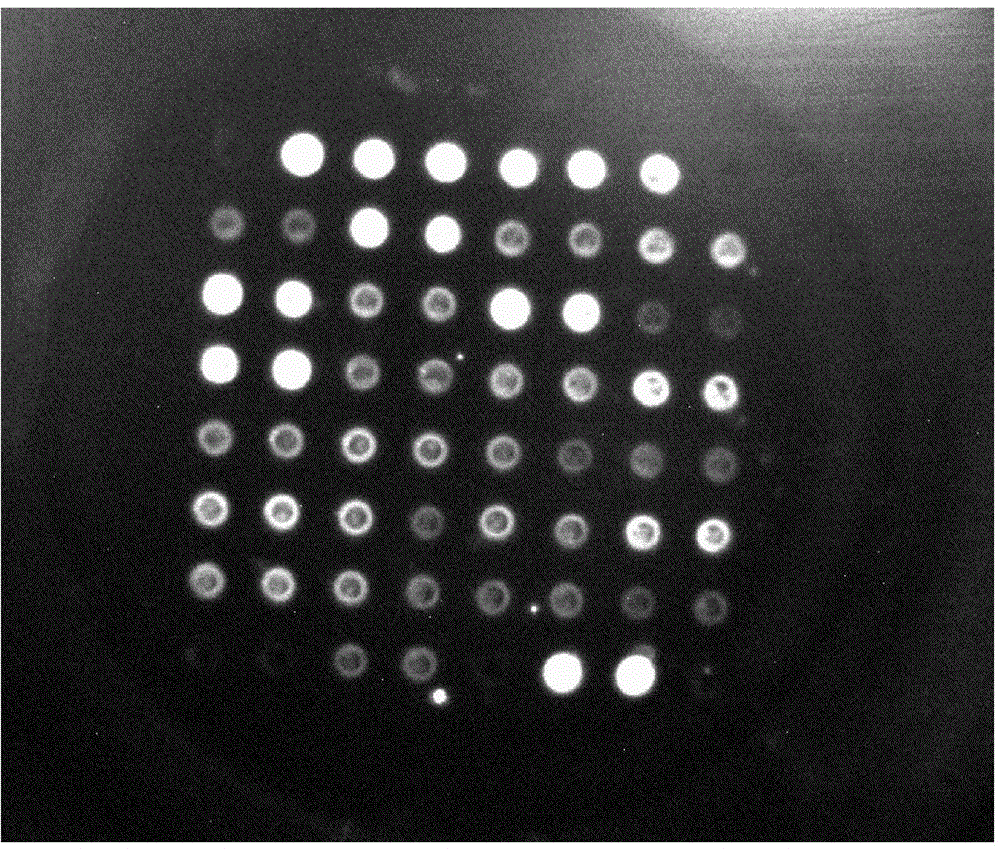
[0032] Example 1: Screening of Trace Antibodies in the Supernatant Stage of Hybridoma Cells
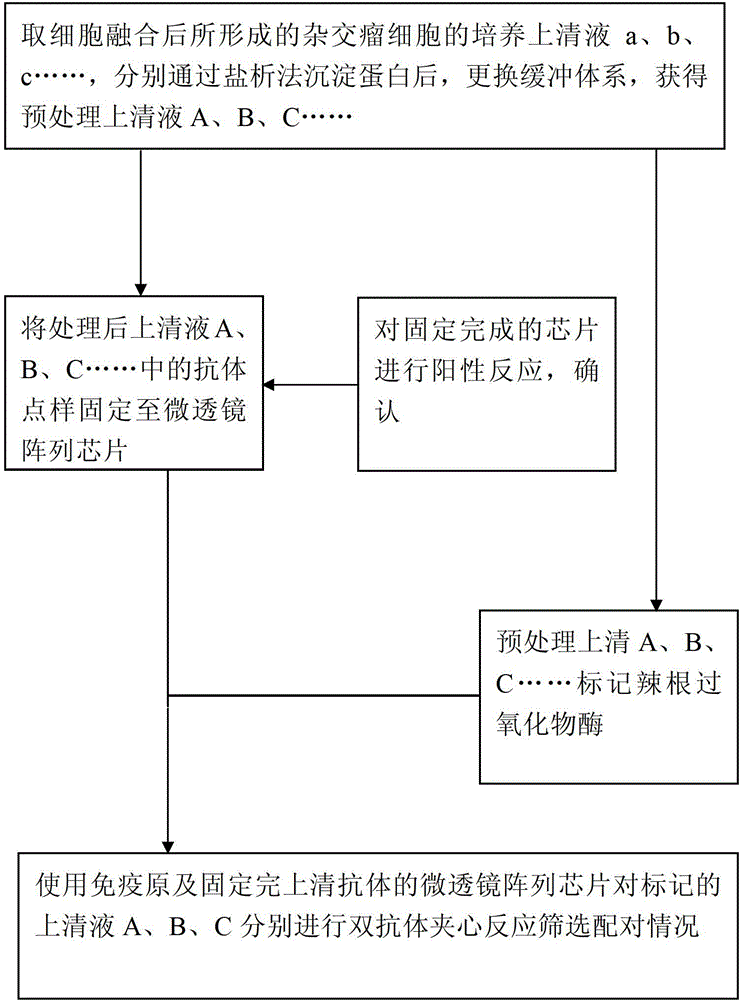
[0033] 1. Take the culture supernatant of the hybridoma cells formed after cell fusion, remove impurities by salting out and replace the buffer system to obtain the pretreatment supernatant. The specific steps are as follows:
[0034] a) Add equal volumes of saturated ammonium sulfate solution to 29 cell supernatants, precipitate at 4°C for 15 hours, centrifuge at 12000 g for 10 min, and discard the supernatant to obtain the precipitate;
[0035] b) The precipitates were dissolved in phosphate buffer with a concentration of 0.01mol / L and a pH of 7.2, respectively, and placed in millipore ultrafiltration tubes with a volume of 0.5ml and a molecular weight of 50KD, centrifuged at 12000g for 10min, and repeated ultrafiltration for 2 times to obtain the pretreatment supernatant;
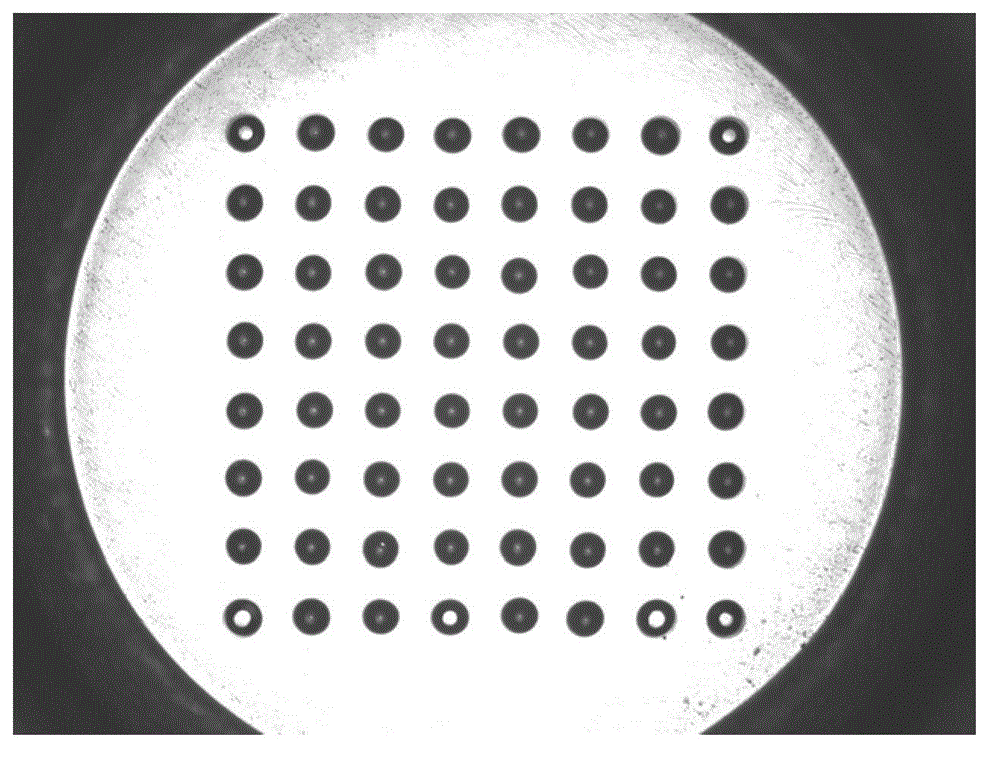
[0036] 2. Immobilize the supernatant antibody pretreated in step 1 to the microlens array chip:
[0037] a)...
Embodiment 2
[0052] 1. Take the culture supernatant of hybridoma cells formed after cell fusion, remove impurities by salting out and replace the buffer system to obtain the pretreatment supernatant:
[0053] a) 20 parts of cell supernatant were added to 150% saturated ammonium sulfate solution of the supernatant volume respectively, precipitated at 4°C for 8 hours, centrifuged at 12000g for 10min, discarded the supernatant to obtain the precipitate;
[0054] b) The precipitates were dissolved in phosphate buffer with a concentration of 0.01mol / L and a pH of 7.2, respectively, and placed in millipore ultrafiltration tubes with a volume of 0.5ml and a molecular weight of 50KD, centrifuged at 12000g for 10min, and repeated ultrafiltration for 2 Obtain pretreatment supernatant after times;
[0055] 2. Fix the supernatant protein pretreated in step 1 to the microlens array chip, the specific steps are as follows:
[0056] a) The pretreatment supernatant in step 1 is diluted 5 times with a spo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com