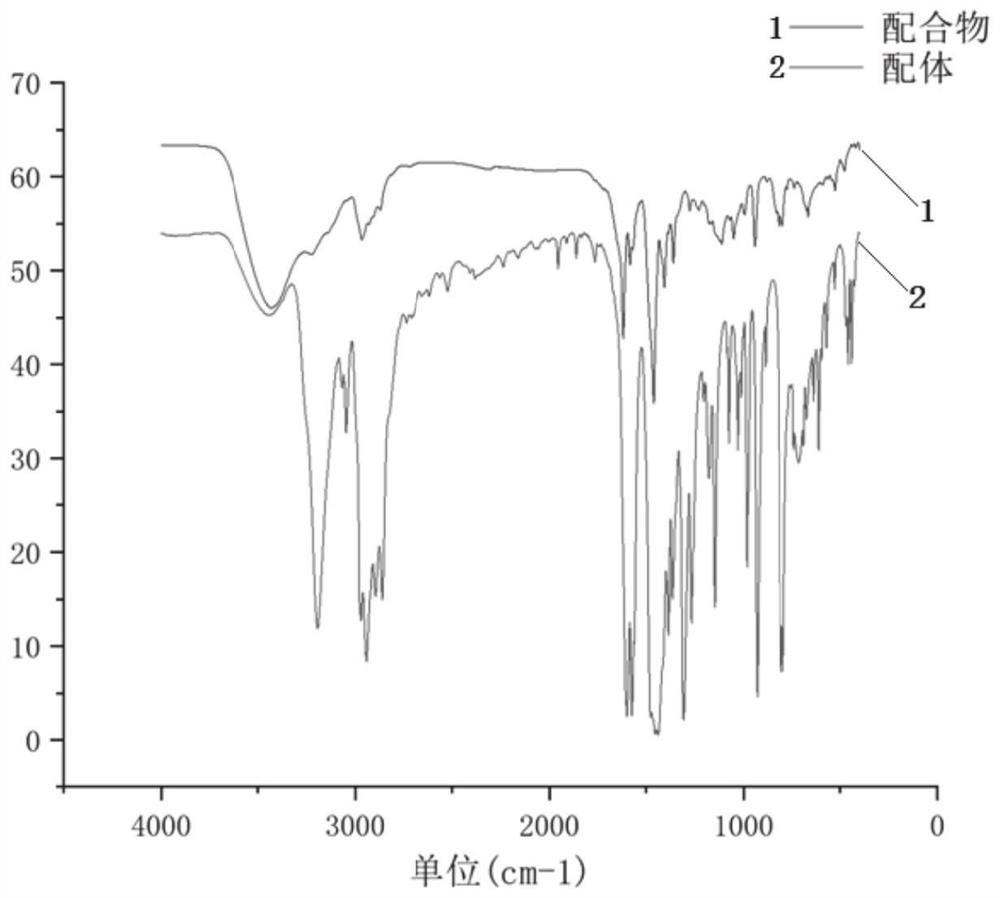
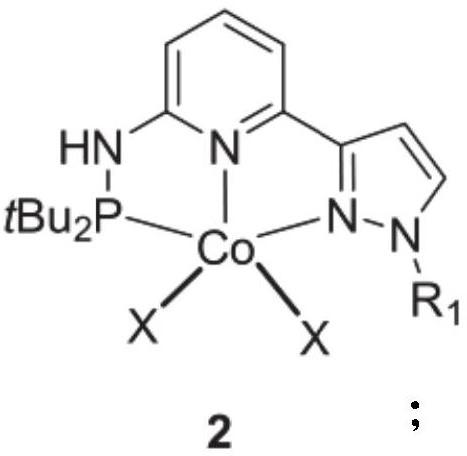
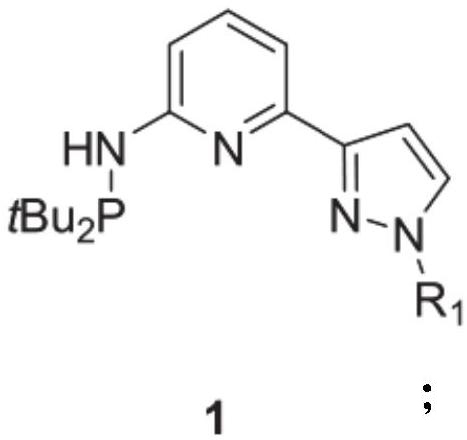
Pyridyl bridged NNP cobalt complex and application thereof
A technology of pyridyl bridge and cobalt complex, which is applied in the direction of cobalt organic compounds, organic compound/hydride/coordination complex catalysts, compounds containing group 8/9/10/18 elements of the periodic table, etc., to achieve synthesis The route is simple, the reaction is green, and the effect of less synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] Under nitrogen, pyridyl-bridged NNP ligand 1a (350.24mg, 1.1mmol), cobalt dichloride (129.8mg, 1mmol) was reacted in 5mL of tetrahydrofuran solvent at 65°C for 24h with stirring. The volatile components were removed under reduced pressure, and the solid was recrystallized from toluene / n-hexane (v / v=1 / 3) to obtain the target product 2a (381.0 mg, yield 85%) as a green solid.
Embodiment 2
[0042] The reaction steps and operations are the same as in Example 1, except that the reaction time of the system is 50 hours. After stopping the reaction, the target product 2a (367.6 mg, yield 82%) was obtained as a green solid after post-treatment. It shows that prolonging the reaction time is not beneficial to increase the yield of the target product.
Embodiment 3
[0044] The reaction steps and operations are the same as in Example 1, except that the reaction solvent is ethanol, and the reaction temperature is 65°C. After stopping the reaction, the target product 2a (363.1 mg, yield 81%) was obtained as a green solid after post-treatment. It shows that this reaction can also be carried out in protic solvents.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com