Anti-folate receptor alpha antibody, its conjugate and use thereof
An anti-folate and conjugate technology, applied in the field of biomedicine, can solve the problems of low side effects, high specificity of monoclonal antibody therapeutic target, limited curative effect, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Preparation of murine anti-FRα monoclonal antibody
[0053] Monoclonal antibodies were prepared by immunizing mice with the extracellular domain protein FOLR1 (abcam) of FRα as an antigen. 0.15ml of FOLR1 protein (75μg protein dissolved in PBS buffer) and 0.15ml of Freund's complete adjuvant (Sigma) were mixed evenly in equal volumes, and Balb / c mice were taken and injected subcutaneously in the back.
[0054] A total of 5 mice were immunized, and each mouse was injected with 0.3 ml. The second injection was performed after a 2-week interval. The amount of antigen injected into each mouse was the same as the first injection. The third injection was performed after a 4-week interval. The amount of antigen injected into each mouse was the same as the first injection. Blood drawn.
[0055] The serum of the mice was detected by enzyme-linked immunosorbent assay (ELISA), and the spleen of the mouse with the highest anti-FOLR1 antibody titer in the serum was taken...
Embodiment 2
[0059] Example 2 Humanization of anti-FRα monoclonal antibodies
[0060] Humanization was performed by grafting the light and heavy chain CDRs of a murine anti-FRα monoclonal antibody into human IgG1 variable regions.
[0061] The heavy chain variable region (VH) of a humanized anti-FRα monoclonal antibody was designed, and an amino acid residue His in the framework region 3 (FWR3) of the human germline heavy chain was converted into a murine isomeric residue Arg, The conformation of the CDRs is preserved in the reconstituted FOLR1-binding antibody.
[0062] At the same time, the light chain variable region (VL) of the humanized anti-FRα monoclonal antibody was designed, and the amino acid residues Val and Tyr in the human germline light chain FWR2 were converted into the mouse isomeric residues Ser and Ser, and the The conversion of amino acid residues Ala and Tyr in the human germline light chain FWR3 to the murine isopositional residues Thr and Phe preserves the conformati...
Embodiment 3
[0069] Example 3 Preparation of Antibody Conjugates
[0070] Prepare reducing buffer: dissolve TCEP (Tris-2-carboxyethyl-phosphine, tris(2-carboxyethyl) phosphine) and DTPA (diethylenetriaminepentaacetic acid, diethylenetriaminepentaacetic acid) in PBS, both substances in reducing buffer The concentrations were 0.26 mM and 2 mM, respectively.
[0071] Antibody reduction: 20 mg / mL mAb (in PBS buffer) was mixed with reducing buffer at a volume ratio of 1:1, and the reaction was stirred at 25° C. for 2 h.
[0072] Preparation of small molecule drug solution: The small molecule toxins MC-VC-PAB-MMAE, MC-VC-PAB-MMAF or MC-MMAF were dissolved in DMSO (dimethyl sulfoxide, dimethyl sulfoxide), respectively, to a final concentration of 10 mM.
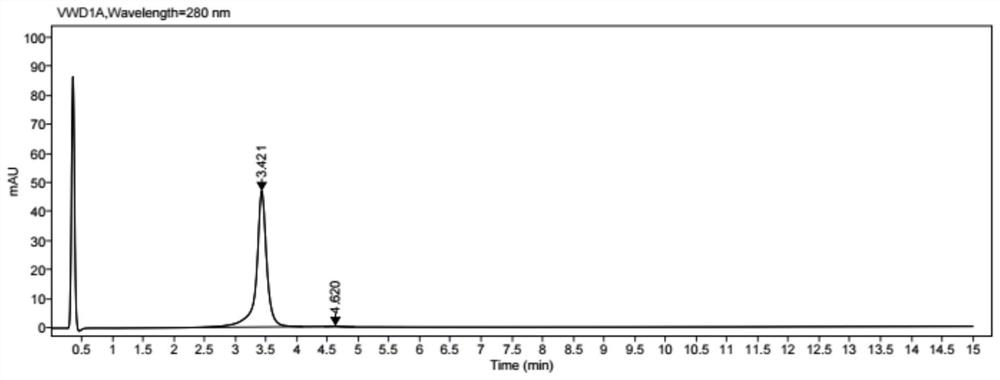
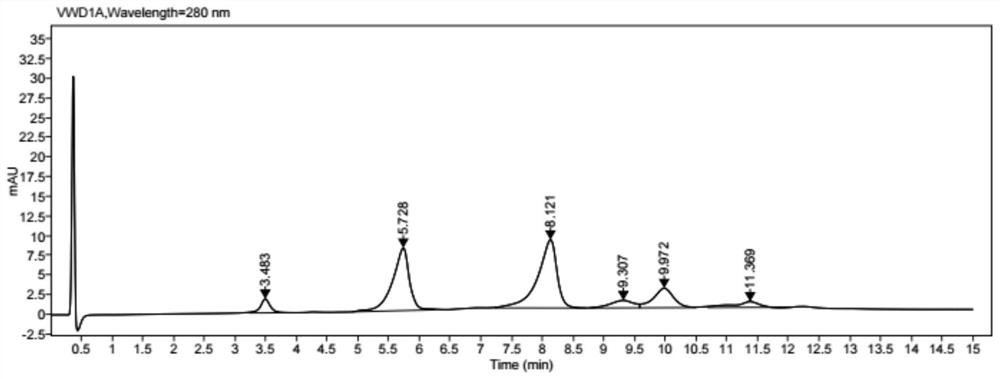
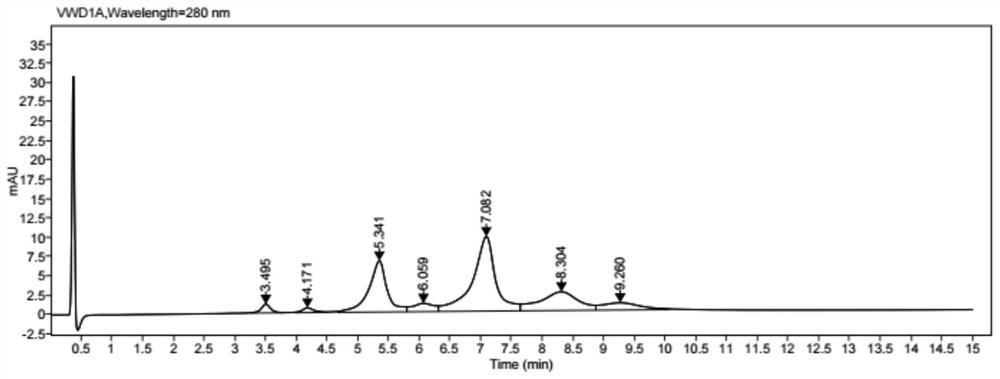
[0073] Coupling: add 25% DMSO to the reduced protein, then slowly add the small molecule drug solution according to the molar ratio of the small molecule drug to the antibody 4.4 for coupling, and stir for 1 h at 25°C. Finally, the conjugated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com