Method for synthesizing dihydrofuran containing 1, 3-indandione spiro skeleton by using micro-channel reaction device
A technology of microchannel reaction and indanedione spiro, which is applied in chemical instruments and methods, chemical/physical/physical chemical reactors, chemical/physical/physical chemical processes, etc., can solve the problem of low reaction efficiency, long reaction time, reaction The steps are cumbersome and other problems, to achieve the effect of speeding up the reaction rate, green reaction conditions, and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
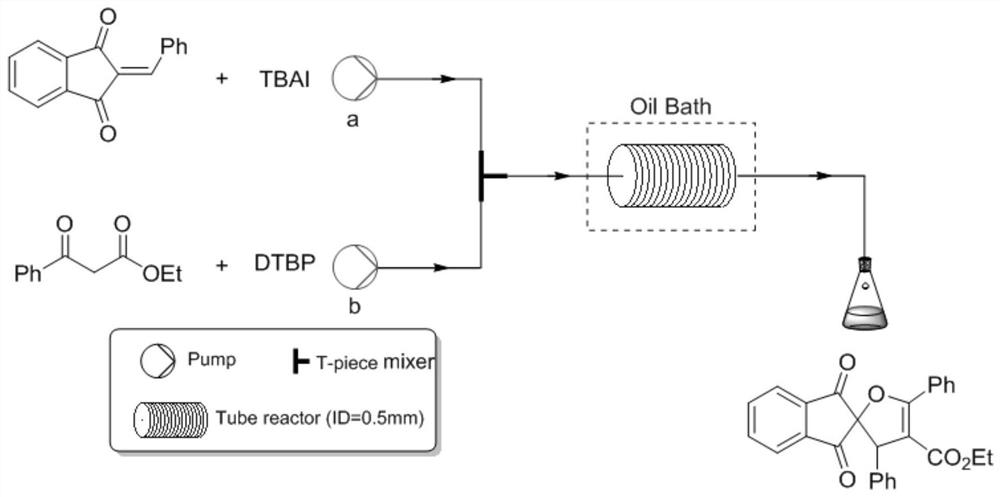
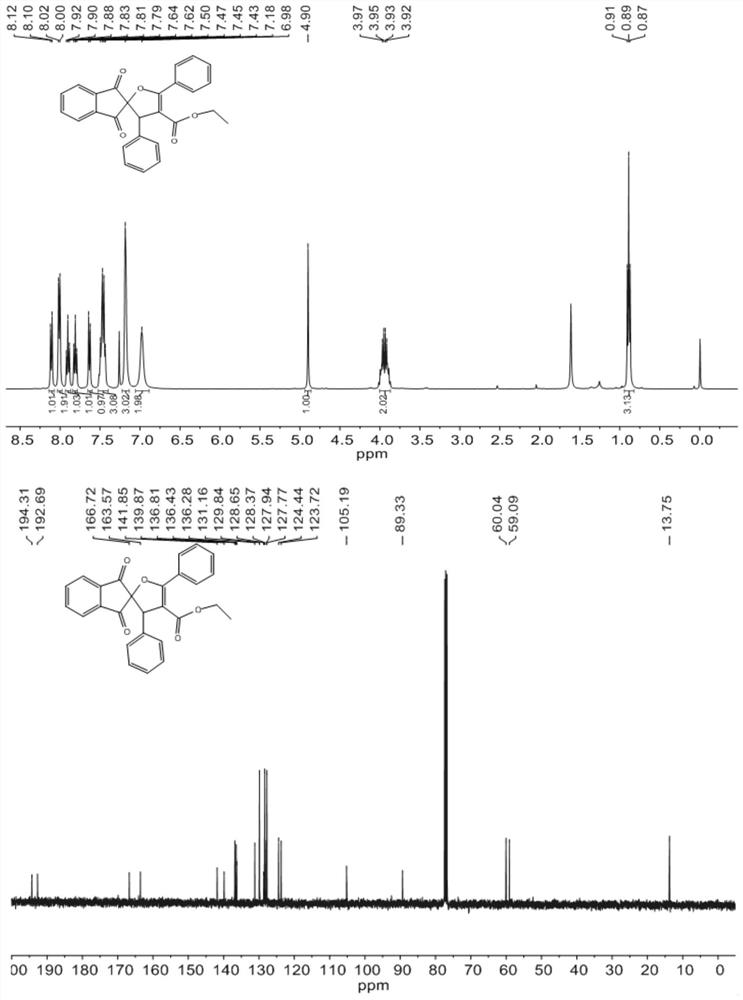
[0043] Dissolve 1mmol (0.234g) of 2-benzylidene indene-1,3-dione and 0.2mmol (0.074g) of tetrabutylammonium iodide in 10ml of γ-valerolactone, and the resulting mixed solution is denoted as Solution A; 1.2mmol (0.23g) of ethyl benzoylacetate, 3mmol (0.44g) of tert-butyl peroxide are dissolved in 10ml of γ-valerolactone, and the resulting mixed solution is designated as solution B, and then Solution A and solution B are pumped into the microchannel reaction device according to the flow volume ratio of 1:1, and the flow rate is 0.5mL / min respectively, and enter the microchannel reactor after being mixed by the Y-type mixer (the polytetrafluoroethylene The inner diameter of the tube is 0.5 mm, and the volume of the polytetrafluoroethylene tube is 10 mL) at 120° C. for 10 min. The organic phase was obtained from the discharge of the microreactor, concentrated in vacuo to obtain the crude product, and separated by column chromatography with a developing agent with a ratio of petrol...
Embodiment 2
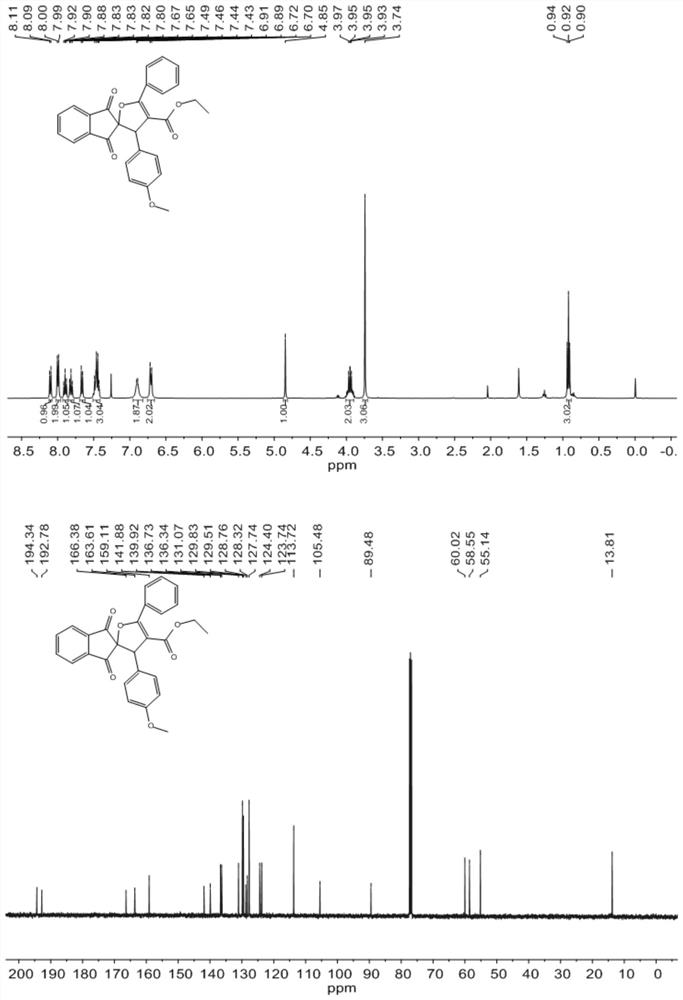
[0045] Dissolve 1mmol (0.264g) of 2-(4-methoxybenzylidene)-1H-indene-1,3(2H)-dione, 0.2mmol (0.074g) of tetrabutylammonium iodide in 10ml of In γ-valerolactone, the resulting mixed solution is designated as solution A; 1.2 mmol (0.23 g) of ethyl benzoyl acetate and 3 mmol (0.44 g) of tert-butyl peroxide are dissolved in 10 ml of γ-valerolactone In the ester, the resulting mixed solution is recorded as solution B, and then solution A and solution B are pumped into the microchannel reaction device according to the flow volume ratio of 1:1, the flow rate is 0.5mL / min, and enter the microchannel reaction after being mixed by a Y-shaped mixer. React at 120° C. for 10 min in a microreactor (the inner diameter of the polytetrafluoroethylene tube of the microreactor is 0.5 mm, and the volume of the polytetrafluoroethylene tube is 10 mL). The organic phase was obtained from the discharge of the microreactor, concentrated in vacuo to obtain the crude product, which was separated by deve...
Embodiment 3
[0047] Dissolve 1mmol (0.268g) of 2-(3-chlorobenzylidene)-1H-indene-1,3(2H)-dione, 0.2mmol (0.074g) of tetrabutylammonium iodide in 10ml of γ - In valerolactone, the resulting mixed solution is denoted as solution A; 1.2mmol (0.23g) of ethyl benzoylacetate, 3mmol (0.44g) of tert-butyl peroxide are dissolved in 10ml of γ-valerolactone , the resulting mixed solution is recorded as solution B, then solution A and solution B are pumped into the microchannel reaction device according to the flow volume ratio of 1:1, the flow rate is 0.5mL / min, and enter the microchannel reactor after being mixed by a Y-type mixer In the medium (the inner diameter of the polytetrafluoroethylene tube of the microreactor is 0.5 mm, and the volume of the polytetrafluoroethylene tube is 10 mL), react at 120° C. for 10 min. The organic phase was obtained from the discharge of the microreactor, concentrated in vacuo to obtain the crude product, and separated by column chromatography with a developing agen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com