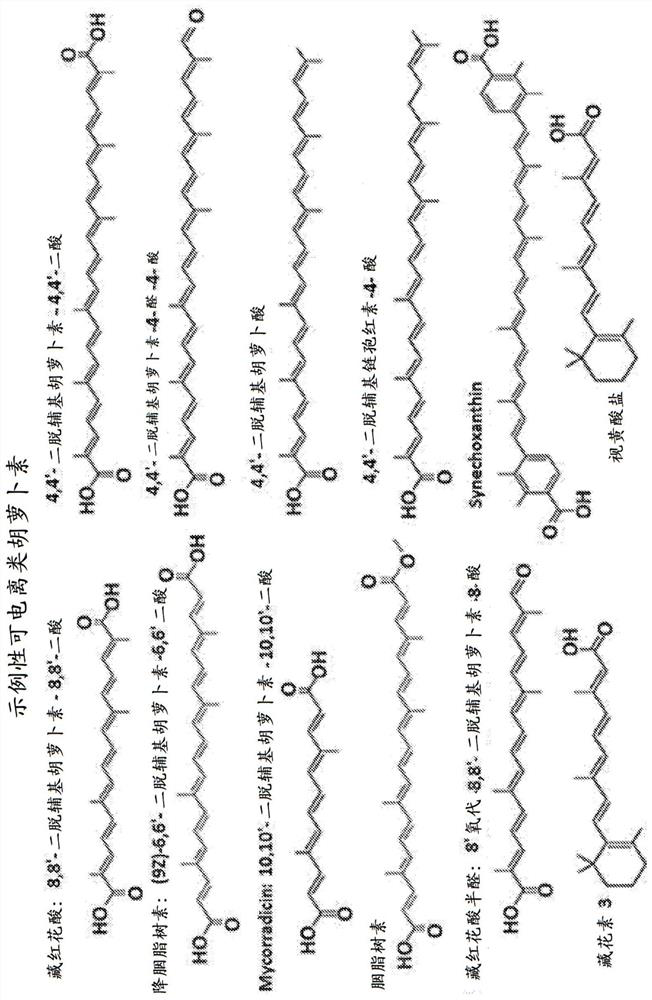
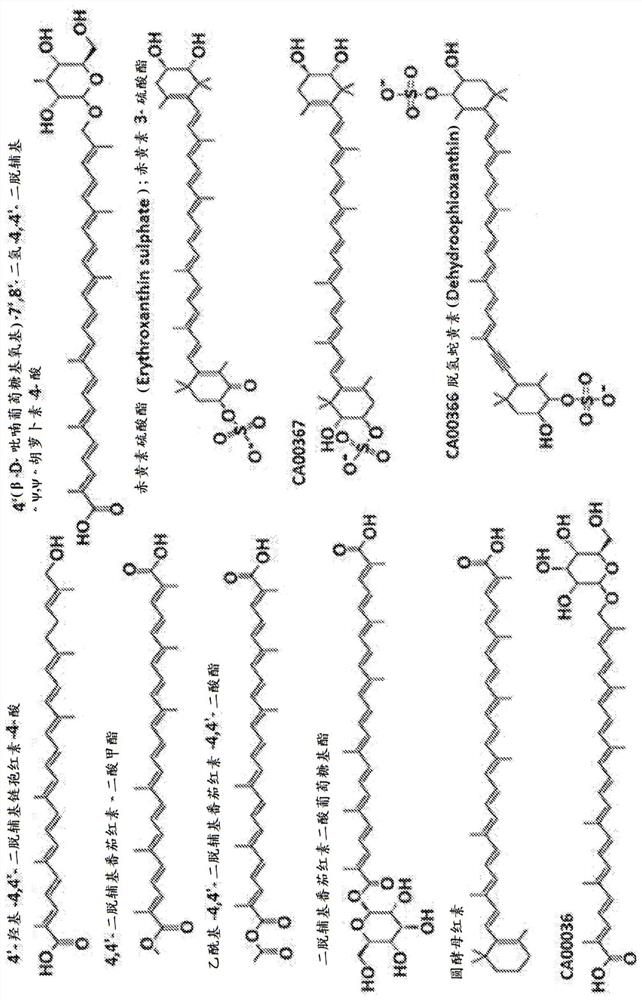
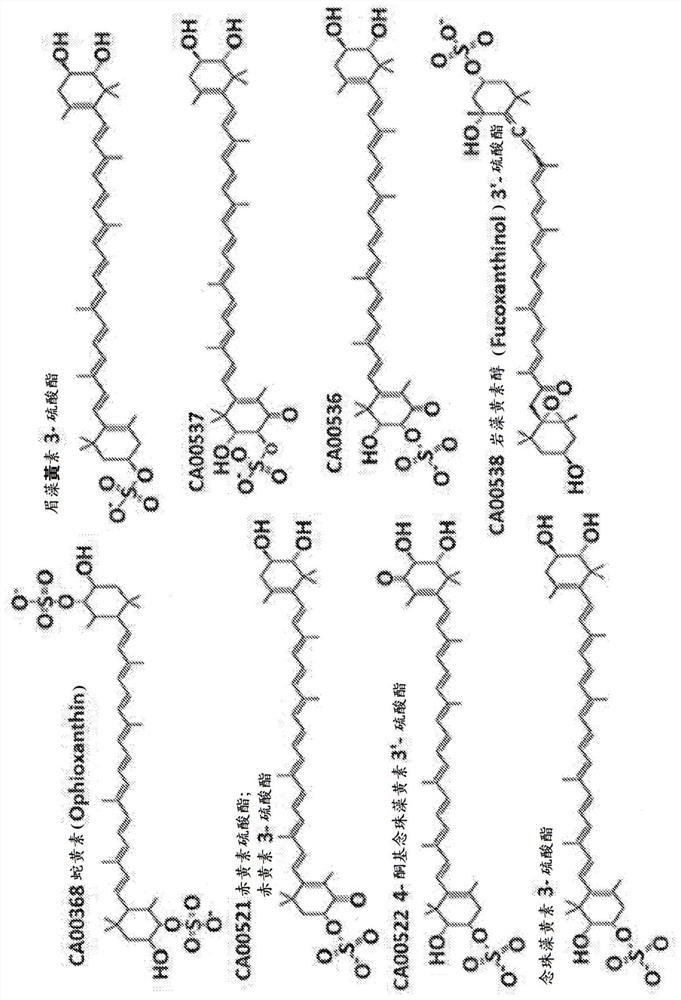
Carotenoid compositions and uses thereof
A technology of carotene and composition, applied in the field of delivery and use of pharmaceutical composition, preparation, delivery and use of composition, can solve the problems such as not showing clinical therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0518] Embodiment 1-trans-production of calcium crocetin liposome
[0519] Trans-crocetin liposomes were produced using two different variants of trans-crocetin, namely: trans-crocetin free acid (TC) and its sodium salt trans-sodium crocetin (STC). Trans-crocetin was encapsulated in liposomes by the following procedure.
[0520] Multiplex bilayer (multilamellar) vesicle (MLV) production :
[0521]First, the lipid components of the liposome lipid membrane are weighed out and combined as a concentrated solution in ethanol at a temperature of about 65°C. In one preparation, the lipids used were hydrogenated soybean phosphatidylcholine, cholesterol, and DSPE-PEG-2000 (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methyl Oxy(polyethylene glycol)-2000]). The molar ratio of HSPC:cholesterol:PEG-DSPE is about 3:2:0.15. In another preparation, the lipids used were HSPC, cholesterol, PEG-DSPE-2000 and 1-palmitoyl-2-glutaryl-sn-glyceryl-3-phosphocholine (PGPC). The molar rati...
Embodiment 2
[0532] Example 2 - with Preparation of calcium acetate liposomes
[0533] Liposomes loaded with calcium acetate were prepared by the following procedure. First, the lipid components of the liposome lipid membrane are weighed out and combined as a concentrated solution in ethanol at a temperature of about 65°C. In one example, the lipids used were hydrogenated soybean phosphatidylcholine, cholesterol, and DSPE-PEG-2000 (1,2-distearoyl-sn-glyceryl-3-phosphoethanolamine-N-[methoxy -(polyethylene glycol)-2000]).
[0534] The molar ratio of HSPC:cholesterol:PEG-DSPE is about 3:2:0.15. In another example, the lipids used are HSPC, cholesterol, PEG-DSPE-2000, and 1-palmitoyl-2-glutaryl-sn-glyceryl-3-phosphocholine (PGPC). The molar ratio of HSPC:cholesterol:PEG-DSPE:PGPC is about 2.7:2:0.15:0.3.
[0535] Next, calcium acetate was dissolved in aqueous buffer at a concentration of 125 or 250 mM, pH 7.0. The calcium acetate solution was heated to 65°C. The ethanol lipid solution...
Embodiment 3
[0536] Example 3-MTC liposome production and characterization
[0537] Production of trans-crocetin liposomes using a magnesium acetate gradient:
[0538] For the production of trans-magnesium crocetin liposomes, two different molecular variants can be used, namely: trans-crocetin free acid (TC) and its sodium salt, trans-sodium crocetin (STC).
[0539] Liposomes containing magnesium acetate were prepared by the following procedure. First, the lipid components of the liposome membrane are weighed out and combined as a concentrated solution in ethanol at a temperature of about 65°C. In one example, the lipids used were hydrogenated soybean phosphatidylcholine, cholesterol, and DSPE-PEG-2000 (1,2-distearoyl-sn-glyceryl-3-phosphoethanolamine-N-[methoxy -(polyethylene glycol)-2000]). The molar ratio of HSPC:cholesterol:PEG-DSPE is about 3:2:0.15. In another example, the lipids used are HSPC, cholesterol, PEG-DSPE-2000, and 1-palmitoyl-2-glutaryl-sn-glyceryl-3-phosphocholine ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| zeta potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com