Aceclofenac synthesis refining process
A technology of aceclofenac and process, applied in the field of raw material drug synthesis, can solve the problems of many by-products, the purity of aceclofenac needs to be improved, etc., and achieve the effects of improving whiteness and improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
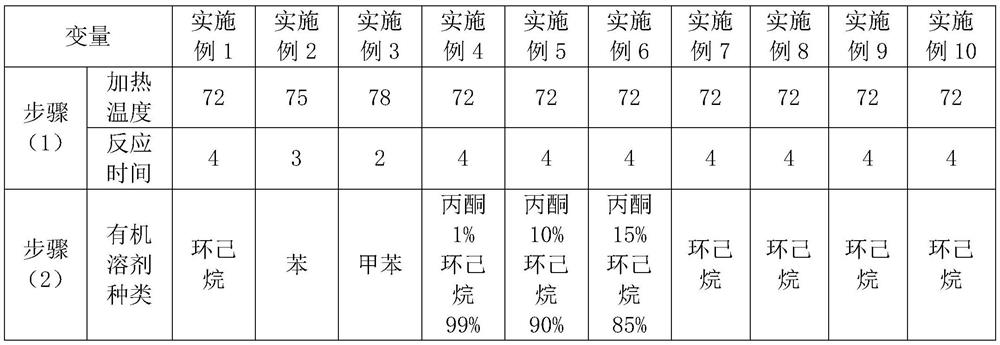
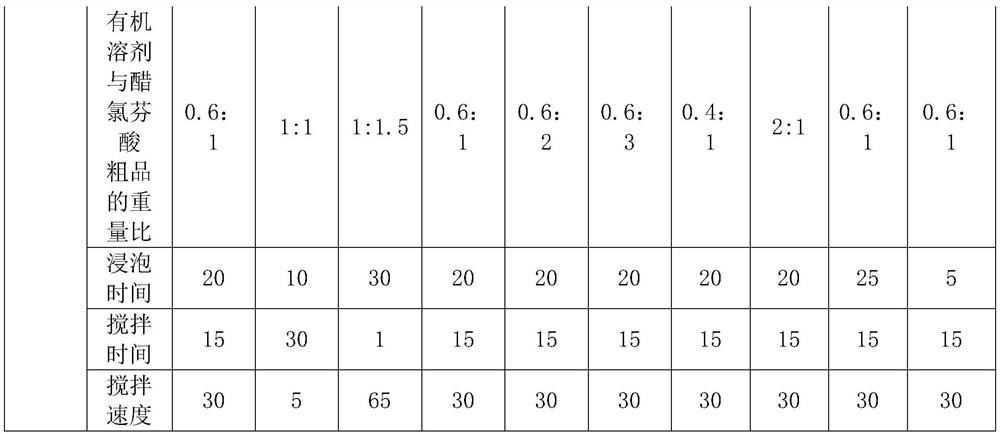
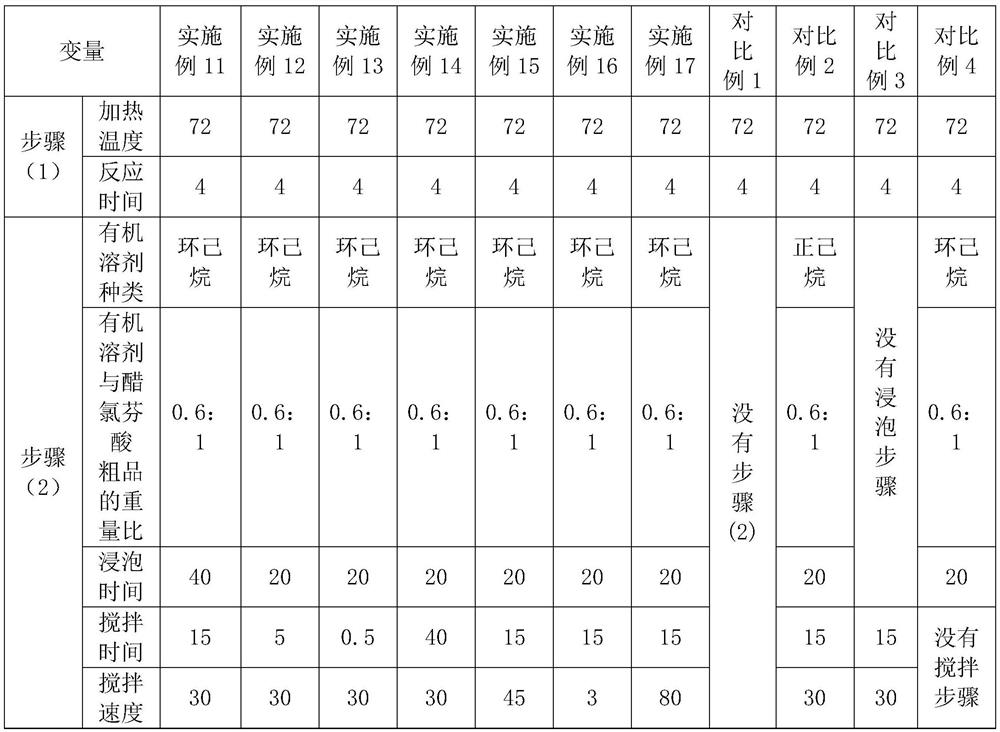
[0032] A process for synthesizing and refining aceclofenac, comprising the following steps:
[0033] (1) Add 80ml of formic acid and 40g of tert-butyl aceclofenac to a three-necked flask, heat up to 72°C and keep warm, react for 4 hours, cool to normal temperature, filter with suction, wash with water, rinse in a centrifuge until the pH of the drain Above 5.0, centrifuge and dry to obtain 33g aceclofenac crude product;
[0034] (2) Put 33g of aceclofenac crude product into a beaker, add 19.8ml cyclohexane to cover the aceclofenac crude product, soak for 20min, stir and wash at a stirring speed of 30rpm, stirring time is 15min, centrifuge to dry, and dry at 80°C, Obtain 32.5g of aceclofenac, the refined yield is 98.5%, and the HPLC purity is 99.993%.
Embodiment 2
[0036] A process for synthesizing and refining aceclofenac, comprising the following steps:
[0037] (1) Add 80ml of formic acid and 40g of tert-butyl aceclofenac to a three-necked flask, heat up to 75°C and keep warm, react for 3 hours, cool to normal temperature, filter with suction, wash with water, rinse in a centrifuge until the pH of the drain Above 5.0, centrifuge to dry, obtain 32g aceclofenac crude product;
[0038] (2) Put 32g of aceclofenac crude product into a beaker, add 32ml of cyclohexane to cover the aceclofenac crude product, soak for 20min, stir and wash at a stirring speed of 30rpm, stirring time is 15min, spin dry by centrifugation, and dry at 80°C to obtain 31.7g of aceclofenac, the refined yield is 98.8%, and the HPLC purity is 99.985%.
Embodiment 3
[0040] A process for synthesizing and refining aceclofenac, comprising the following steps:
[0041] (1) Add 80ml of formic acid and 40g of tert-butyl aceclofenac to a three-necked flask, heat up to 78°C and keep warm, react for 3 hours, cool to normal temperature, filter with suction, wash with water, rinse in a centrifuge until the pH of the drain Above 5.0, centrifuge to dry, obtain 31g aceclofenac crude product;
[0042] (2) Put 31g of aceclofenac crude product into a beaker, add 46.5ml cyclohexane to cover the aceclofenac crude product, soak for 20min, stir and wash at a stirring speed of 30rpm for 15min, spin dry by centrifugation, and dry at 80°C. Obtain 30.4g of aceclofenac, the refined yield is 98.1%, and the HPLC purity is 99.986%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com