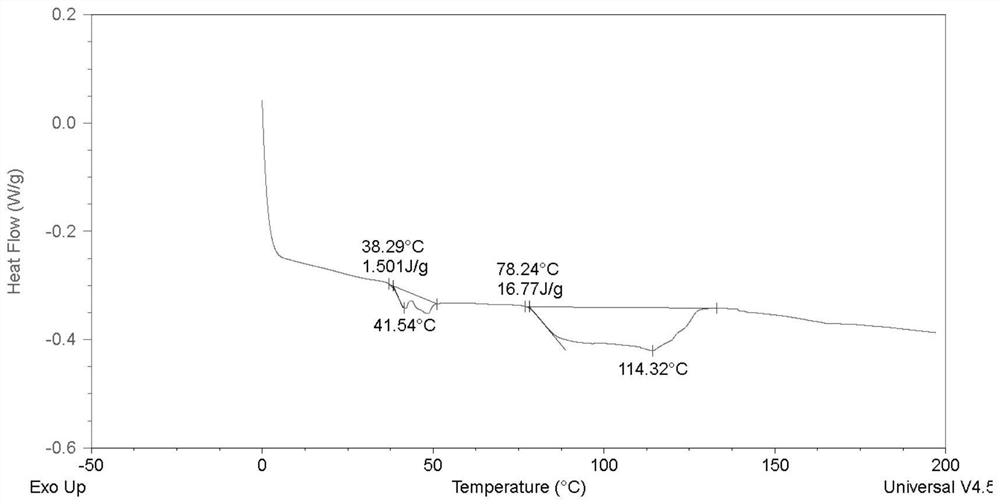
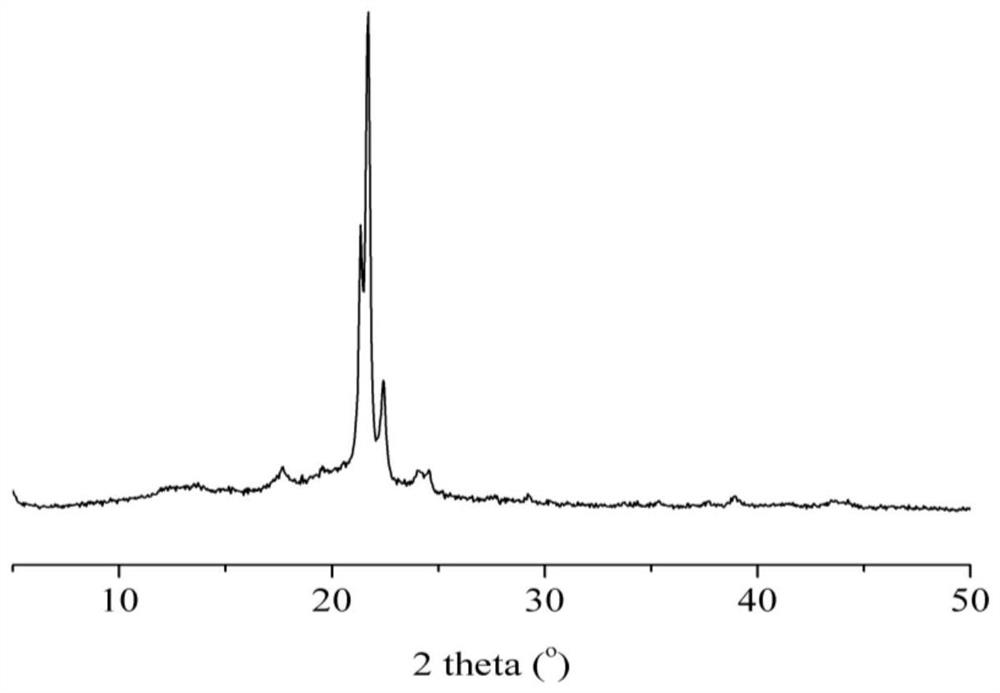
Polyester polyol and preparation method thereof
A polyester polyol and diol technology, applied in the field of polymers, can solve the problems of three wastes, etc., and achieve the effect of saving energy consumption and excellent crystallization performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention provides a kind of preparation method of polyester polyol described in above-mentioned technical scheme, comprising:
[0037] Carrying out the first reaction of PET, poly(carbonate-ether) glycol, small molecule glycol, catalyst and antioxidant to obtain a reaction product;
[0038] The reaction product and the small molecule dibasic acid are subjected to a second reaction to obtain polyester polyol.
[0039] In the present invention, the type and amount of the PET, poly(carbonate-ether) glycol, small molecule glycol, catalyst, antioxidant and small molecule dibasic acid are consistent with those described in the above-mentioned technical scheme. This will not be repeated here.
[0040] In the present invention, the first reaction is preferably carried out under the protection of nitrogen. In the present invention, the first reaction preferably includes:
[0041] After raising the temperature of PET, poly(carbonate-ether) glycol, small molecule gly...
Embodiment 1
[0048] The poly(carbonate-ether) glycol used in embodiment 1 is prepared according to the embodiment 15 method reported in the invention patent 201110231493.6; The poly(carbonate-ether) glycol used in embodiment 2 is according to the report in the invention patent 201110231493.6 Embodiment 9 method is prepared; Poly(carbonate-ether) glycol used in embodiment 3 is prepared according to the embodiment 17 method reported in the invention patent 201110231493.6; Poly(carbonate-ether) glycol used in embodiment 4 is according to The method of Example 12 reported in the patent of invention 201110231493.6 is prepared; the poly(carbonate-ether) glycol used in Example 5 is prepared according to the method of Example 18 reported in the patent of invention 201110231493.6; the poly(carbonate-ether) glycol used in Example 6 is prepared Ether) diols were prepared according to the method of Example 16 reported in the invention patent 201110231493.6.
[0049] The synthesis of embodiment 1 PET p...
Embodiment 2
[0053] The synthesis of embodiment 2 PET polyester polyols
[0054] Under the protection of nitrogen, 350g of PET, 1200g of poly(carbonate-ether) glycol (the number average molecular weight is 6400, and the carbonate unit content is 36.8%), 117g of 1,4-butanediol are successively dropped into the reactor , 1.15g of manganese acetate, 0.26g of IRGANOX1076, after the temperature rises to 170°C, start stirring, react for 0.5 hours, then gradually raise the temperature to 250°C at a heating rate of 5°C / min, continue to react for 3 hours, the acid value is The reaction was stopped at 10 mgKOH / g to obtain a reaction product.
[0055] Reduce the temperature of the obtained reaction product to 120°C, add 59g of 1,6-adipic acid, gradually raise the temperature to 250°C at a heating rate of 5°C / min, and continue the reaction for 2 hours until the acid value is 30mgKOH / g. Start vacuum dehydration, when the acid value is 5mgKOH / g, cool to 100°C and discharge to obtain PET polyester polyo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com