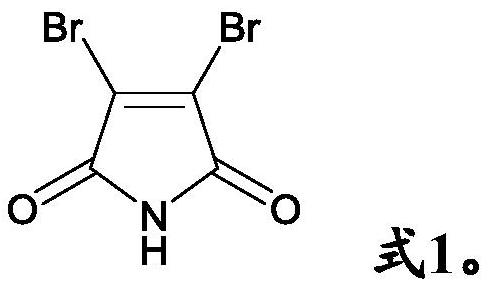
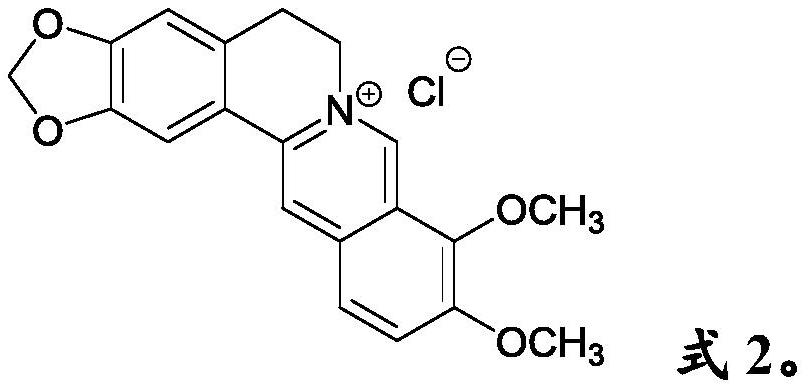
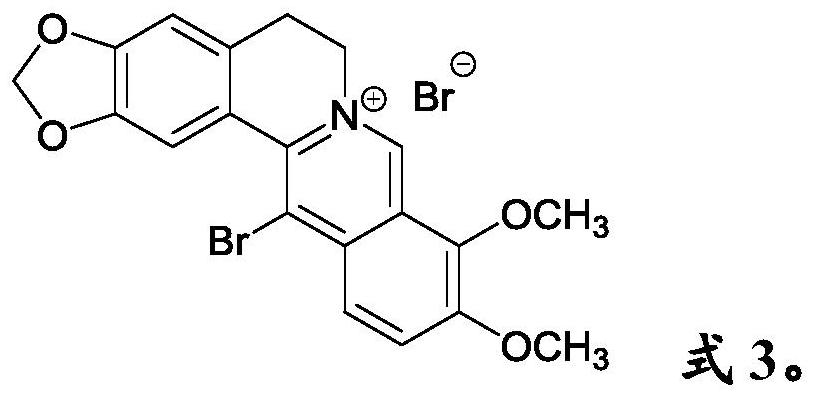
Anti- P.aeruginosa pharmaceutical composition as well as preparation method and application thereof
A technology of Pseudomonas aeruginosa and composition, applied in antibacterial drugs, pharmaceutical formulations, medical preparations containing active ingredients, etc., to achieve the effect of improving antibacterial effect, low price, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The preparation method of the present invention will be further described in detail in conjunction with specific examples below. It should be understood that the following examples are only for illustrating and explaining the present invention, and should not be construed as limiting the protection scope of the present invention. All technologies realized based on the above contents of the present invention are covered within the scope of protection intended by the present invention.
[0051] The experimental methods used in the following examples are conventional methods unless otherwise specified; the reagents and materials used in the following examples can be obtained from commercial sources unless otherwise specified.
Embodiment 1
[0052] Embodiment 1. Berberine and 3,4-dibromomaleimide compatibility
[0053] A solution containing 200 μM berberine and 100 μM 3,4-dibromomaleimide was prepared with pure water or PBS buffer (pH=7.2), and incubated with Pseudomonas aeruginosa in an incubator at 37° C. for 12 hours. Experimental detection bacteriostatic rate ≥ 99.5%.
Embodiment 2
[0054] Embodiment 2. Compatibility of bromoberberine and 3,4-dibromomaleimide
[0055]Use pure water or PBS buffer (pH=7.2) to prepare a solution containing 100 μM bromoberberine and 50 μM 3,4-dibromomaleimide, and incubate with Pseudomonas aeruginosa in an incubator at 37°C for 12 hours . Experimental detection bacteriostatic rate ≥ 99.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| antibacterial rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com