Nucleic acid composition and kit for detecting EGFR gene mutation and detection method of EGFR gene mutation
A technology of nucleic acid composition and kit, which is applied in the field of genetic engineering and can solve problems such as low detection sensitivity and failure to meet actual needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] A kit for detecting EGFR gene mutation is provided, and the composition of the kit is shown in Table 1.
[0115] The kit includes nucleic acid compositions for detecting EGFR gene mutation (as shown in Table 2 and Table 3), dNTPs, PCR reaction solution, enzyme mixture, positive control substance and blank control substance.
[0116] Wherein, the enzyme mixed solution includes the enzyme I mixed solution. Enzyme I mixture is a mixture of Taq enzymes and antibodies without 5' to 3' exonuclease activity. Enzyme II mixture is a mixture of exonuclease activity and antibody from 5' end to 3' end. Among them, the Taq enzyme was purchased from TAKARA Biological Company and Guangzhou Baiwang Biotechnology Co., Ltd., and the antibody was purchased from TOYOBO Company.
[0117] Mutation detection PCR reaction solution includes: 2mM MgCl2, 50mM Tris, pH8.3, 500mg / L BSA, 100μM dNTPs, 0.4μL enzyme I mixture, 0.1μM forward primer, 0.5μM reverse primer , 0.5 μM probe.
[0118] The ...
Embodiment 2
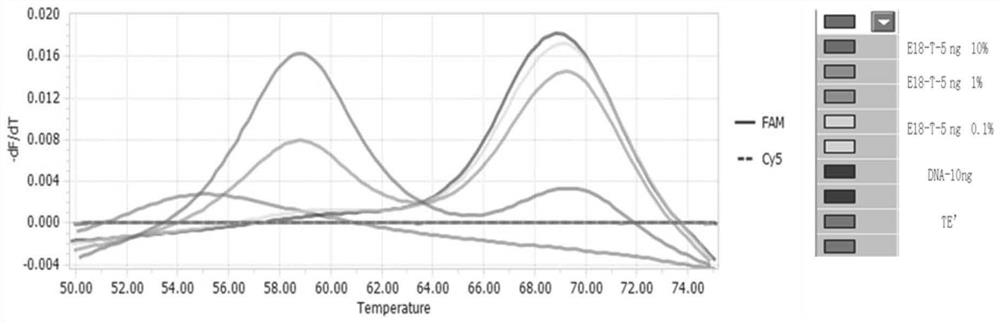
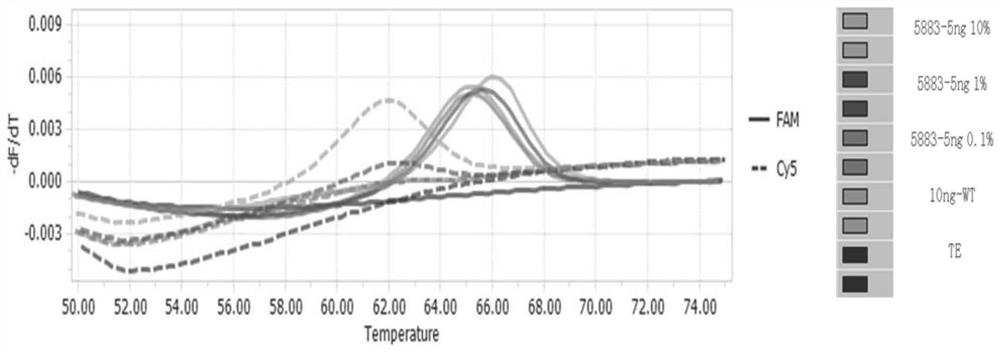
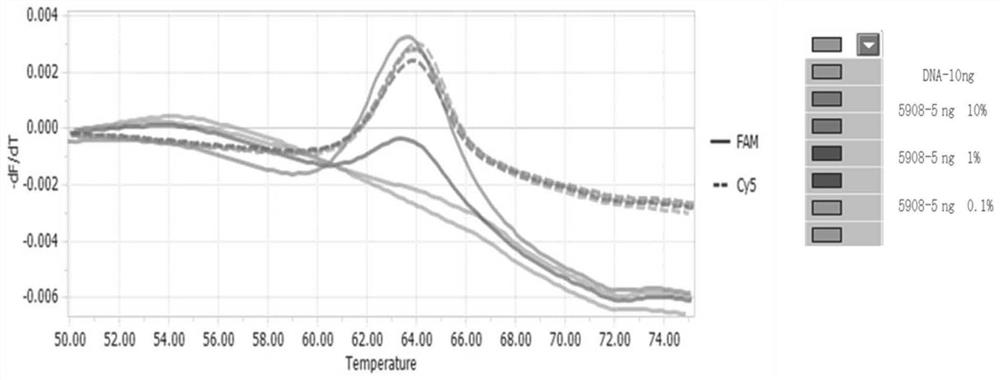
[0131] Adopt the detection limit of the test kit of the detection EGFR gene mutation of embodiment 1
[0132] Take human EGFR wild-type genome, human EGFR mutant gene plasmids and TE buffer buffer, and prepare human EGFR wild-type DNA containing 5ng / μL and human EGFR whose content is 10%, 1% and 0.1% of wild-type respectively For each mutant gene plasmid, record the positive detection rate.
[0133] The specific implementation steps are as follows:
[0134] 1. Extraction of samples to be tested
[0135] Tissue samples were extracted using QIAamp DNA FFPE Tissue Kit from QIAGEN, and plasma samples were extracted using QIAamp Circulating Nucleic Acid Kit from QIAGEN. The extraction process should be carried out in strict accordance with the instructions. The nucleic acid after tissue sample extraction should be diluted to a concentration of 2-100 ng / μL, the concentration of nucleic acid after plasma extraction should not be less than 2 ng / μL, and the purity should satisfy the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com