Use of three components of baical skullcap root for synergistically enhancing cell proliferation promoted by FGF1
A kind of technology of FGF1- and baicalin, applied in the field of molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of Radix Scutellariae Extract
[0029] Purchase dried scutellaria baicalensis slices at the pharmacy, remove impurities, grind the dried scutellaria baicalensis into a uniform powder, weigh 2 g of the powder and add 20 mL of methanol for ultrasonic extraction, 20 min each time, extract 3 times, combine the extracts, and pass the suspension to 0.22 μm filter membrane, blow nitrogen to concentrate to dryness, redissolve in ultrapure water, pass through a 0.22 μm filter membrane and store at 4 °C until use.
Embodiment 2
[0030] Example 2 Screening of active ingredients that bind to FGF1 in Scutellaria baicalensis extract
[0031] 1. Preparation of FGF1-Scutellaria baicalensis active ingredient complex
[0032] Dissolve 1 mg of FGF1 lyophilized powder in 1 ml of ultrapure water to obtain 1 mg / ml of FGF1 aqueous solution, dilute 1 mg / ml of FGF1 with 10 mM pH4 ammonium acetate buffer to 0.1 mg / ml, take 95 ul of FGF1 and 20 mg / ml 5 ul of Scutellaria baicalensis extract was mixed to form 100 ul of FGF1-Scutellaria baicalensis active ingredient mixture; incubate at 37 degrees Celsius for 30 min to make the active ingredients in Scutellaria baicalensis extract combine with FGF1 to form a FGF1-Scutellaria baicalensis active ingredient complex.
[0033] 2. Multi-chamber electrophoresis separation
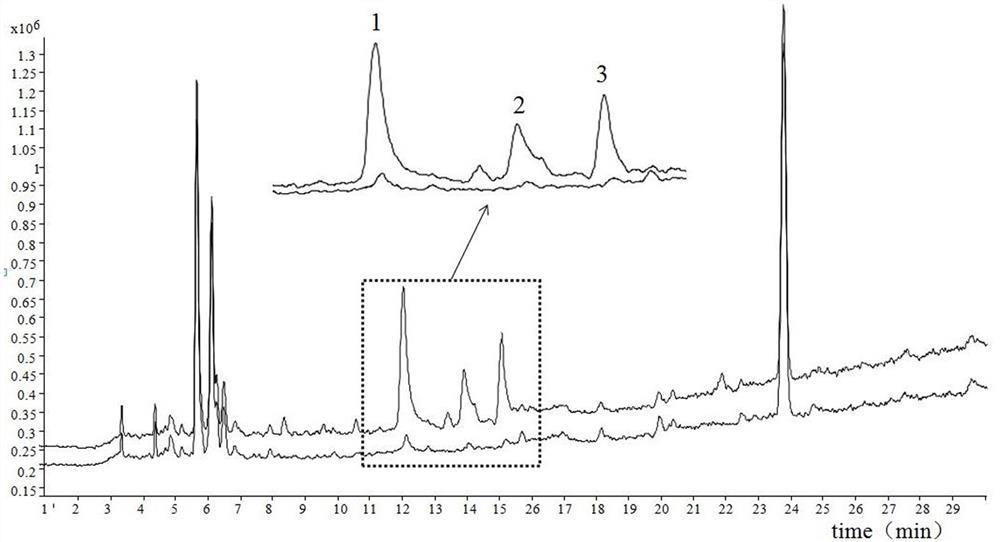
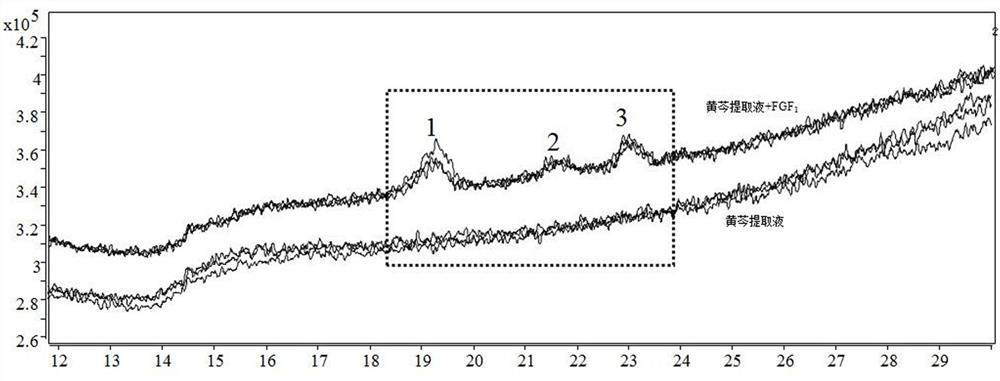
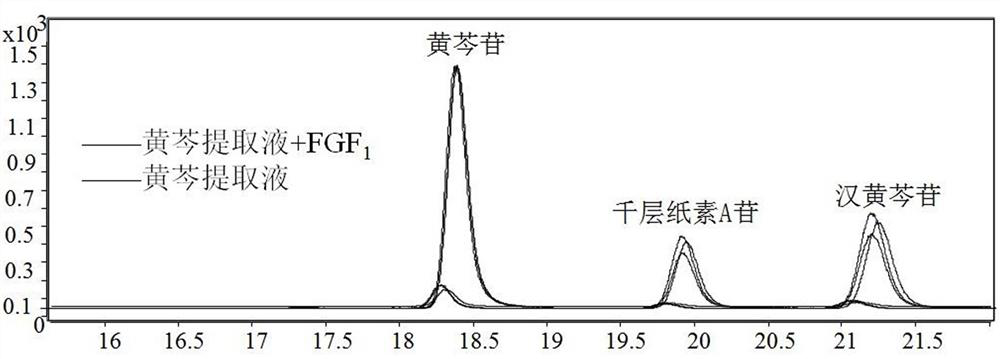
[0034] After hatching, the FGF1-scutellaria baicalensis active ingredient mixed solution is used as the sample solution, and the scutellaria baicalensis extract is used as the control solution, and is divid...
Embodiment 3
[0037] Example 3 Qualitative analysis of active components of Scutellaria baicalensis combined with FGF1
[0038] 1. The solution in the receiving chamber after the dissociation of embodiment 2 is subjected to high performance liquid phase tandem mass spectrometry analysis;
[0039] Chromatographic conditions:
[0040] Chromatographic column: Tnature C18 reverse chromatographic column (4.6 mm×150mm, 5μm)
[0041] Mobile phase: mobile phase A is 0.1% formic acid aqueous solution, mobile phase B is 0.1% formic acid acetonitrile solution;
[0042] Flow rate: 0.5mL / min;
[0043] Injection volume: 5 μL;
[0044] The gradient elution program is as follows: 0-10 min, 25%-40%B; 10-20 min, 40%-65%B; 20-30 min, 65%-80%B;
[0045] Mass Spectrometry Conditions:
[0046] Mass Spectrometry Conditions Electrospray ionization source, negative ion mode, full scan mode; spray gas pressure: 30 psi; drying gas (N2) flow rate: 13.0 L / min, drying gas temperature: 300°C; capillary voltage: 2500...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com