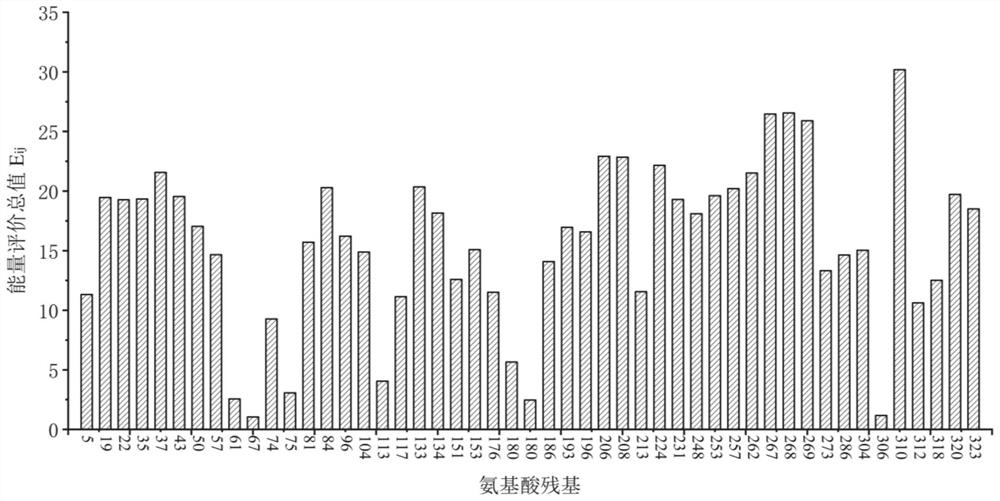
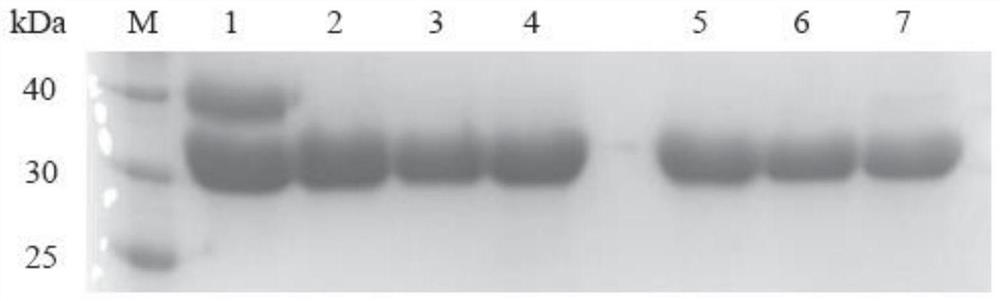
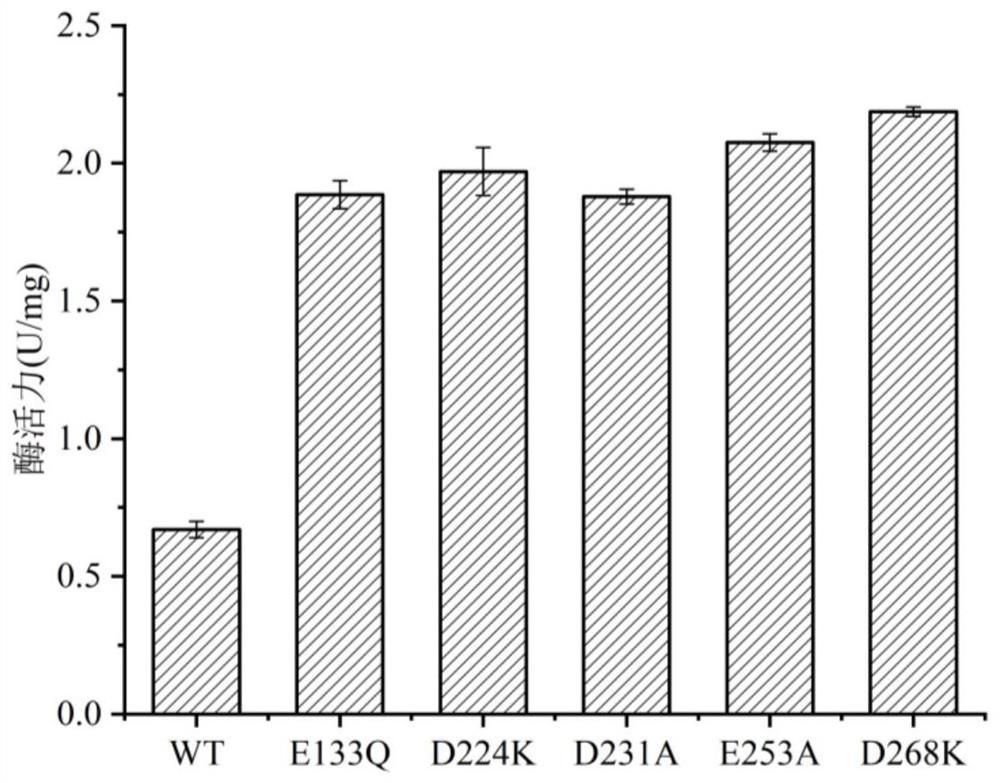
Omega-transaminase mutant and application thereof
A transaminase and mutant technology, which is applied to ω-transaminase mutants and their application fields to achieve the effects of increasing the probability of positive mutation, improving experimental efficiency and feasibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Materials and reagents
[0031] The whole gene of (R)-ω-TA was synthesized by Anhui General Biological Company, and the recombinant plasmid pET-28a-ω-TA was preserved by our laboratory. PrimeSTAR Max DNA polymerase was purchased from TaKaRa Company; Dpn I was purchased from Thermo Scientific Company; SanPrep column plasmid DNA mini-extraction kit, isopropyl-b-D-thiogalactoside (IPTG), kanamycin sulfate (Kanamycin sulfate), pyrodoxal phosphate (pyrodoxal-5'-phosphate, PLP), and improved Bradford protein concentration assay kit were purchased from Sangon Bioengineering (Shanghai) Co., Ltd.; DNA Marker, Protein Marker, E.coli BL21 (DE3), E.coli DH5α, and Ni-NTA chromatography media were purchased from Beijing Quanshijin Biotechnology Co., Ltd.; dansyl chloride (DNS-C1) was purchased from Sigma (USA); γ-aminobutyric acid standard ( γ-aminobutyric acid) was purchased from Fluka (Switzerland); sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com