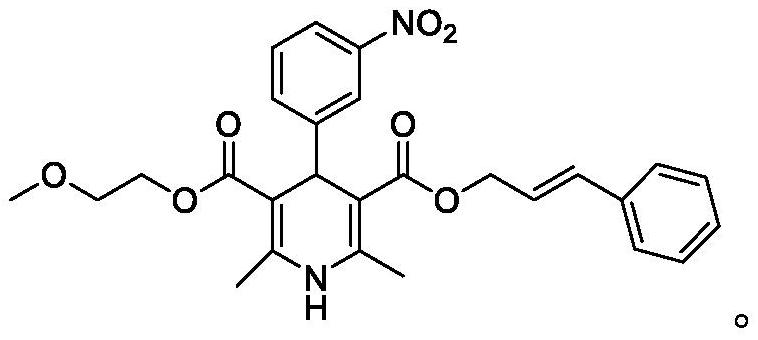
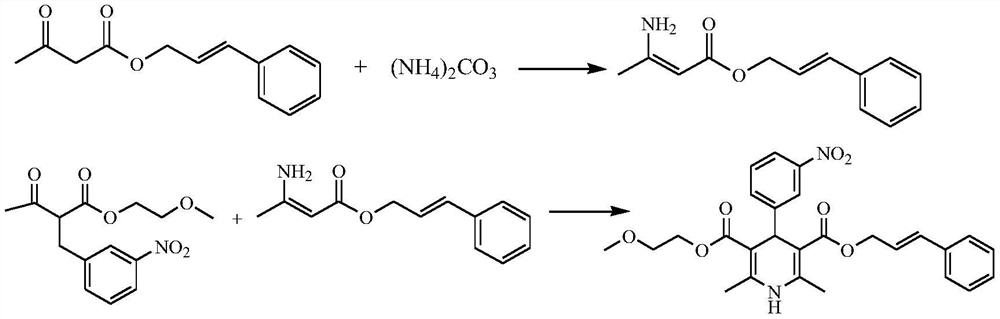
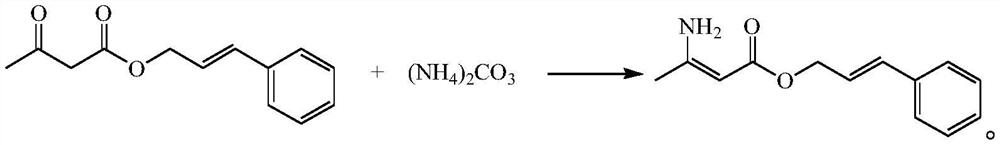Preparation method of cilnidipine
A technology of cilnidipine and crotonic acid, which is applied in the field of compound synthesis, can solve the problems of increasing the impurity content, long reaction time, increase reaction cost and the like, and achieve the effects of improving purity and yield, short reaction time and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] This example provides a preparation method of cinnamyl 3-amino-2-butenoate.
[0045] Add 100 g of cinnamyl acetoacetate into 200 mL of ethanol and stir to dissolve, add 44.0 g of solid ammonium carbonate, stir at a controlled temperature of 25°C, react for 24 hours, filter and wash the filter residue with a small amount of ethanol, collect 274 g of the filtrate, and obtain 3-amino-2- Reaction solution of cinnamyl crotonate.
[0046] The purity detected by liquid phase HPLC is ≥99.01%, and the content of cinnamyl 3-amino-2-butenoate is 0.348 g / g.
Embodiment 2
[0048] This example provides a preparation method of cinnamyl 3-amino-2-butenoate.
[0049] Add 100 g of cinnamyl acetoacetate into 200 mL of isopropanol and stir to dissolve, add 44.0 g of solid ammonium carbonate, stir at a controlled temperature of 20°C, react for 24 hours, filter and wash the filter residue with a small amount of isopropanol, collect 307 g of the filtrate, and obtain 3- A reaction solution of cinnamyl amino-2-butenoate.
[0050] The purity detected by liquid phase HPLC is ≥98.8%, and the content of cinnamyl 3-amino-2-butenoate is 0.296 g / g.
Embodiment 3
[0052] This embodiment provides a preparation method of cilnidipine.
[0053] Add 118.0 g of methoxyethyl 2-(3-nitrophenylidene) acetoacetate to 200 mL of ethanol and stir to dissolve to obtain solution 2, and then add 300 mL of the reaction solution obtained in Example 1 to the mixer through a high-pressure constant-flow pump Inside (the flow rate ratio of the reaction solution to solution 2 is 1.2:1), enter the microchannel reactor (thickness 2mm, 5 groups of microtubes, net volume 43.5mL) through the mixer at a flow rate of 5mL / min, the microchannel The temperature of the reactor was set at 50°C, and the reaction liquid was collected; the reaction liquid was cooled to -20°C to crystallize under stirring, and the crystallization was completed, filtered, and the solid was dried under reduced pressure at 50°C to obtain 181.1 g of cilnidipine finished product, with a yield of 91.4%. Phase purity 99.64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com