Anti-human YKL-40 neutralizing monoclonal antibody as well as preparation and application thereof
A technology of YKL-40, monoclonal antibody, applied in the field of medical bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: YKL-40 protein antigen screening
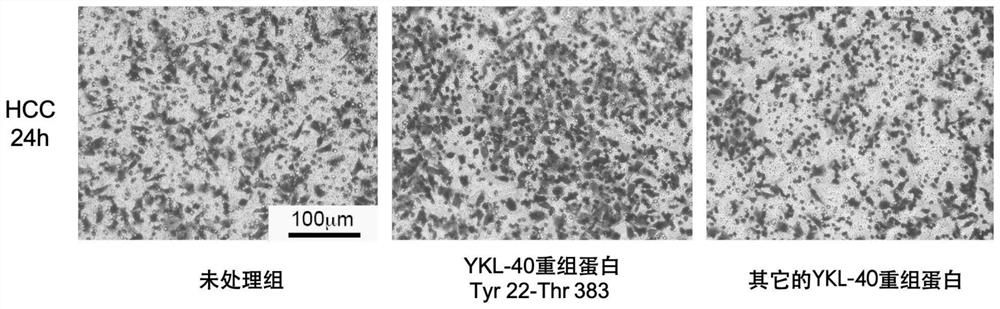
[0038] Antigen screening: Human YKL-40 recombinant proteins from different merchants were selected, and the transwell cell migration system was used to detect the effect of the protein on the migration ability of liver cancer cell MHCC-LM3, and different YKL-40 recombinant proteins were added to the lower chamber of the transwell, and the screening criteria In order to significantly promote the migration of liver cancer cells MHCC-LM3. according to figure 2 , it can be seen that different YKL-40 recombinant proteins have different effects on the migration of liver cancer cells. The YKL-40 recombinant protein we selected can significantly promote the migration ability of liver cancer cells. The antigenic sequence of the YKL-40 recombinant protein is Tyr 22-Thr 383 Structural domain, which has been verified by experiments to be a functional domain that can play a chemotaxis role in liver cancer cells.
Embodiment 2
[0039] Example 2: Application of anti-human YKL-40 neutralizing monoclonal antibody in inhibiting lung metastasis of liver cancer cells
[0040] 1) Neutralizing monoclonal antibody can significantly reduce the concentration of YKL-40 in the serum of patients with lung metastasis of liver cancer
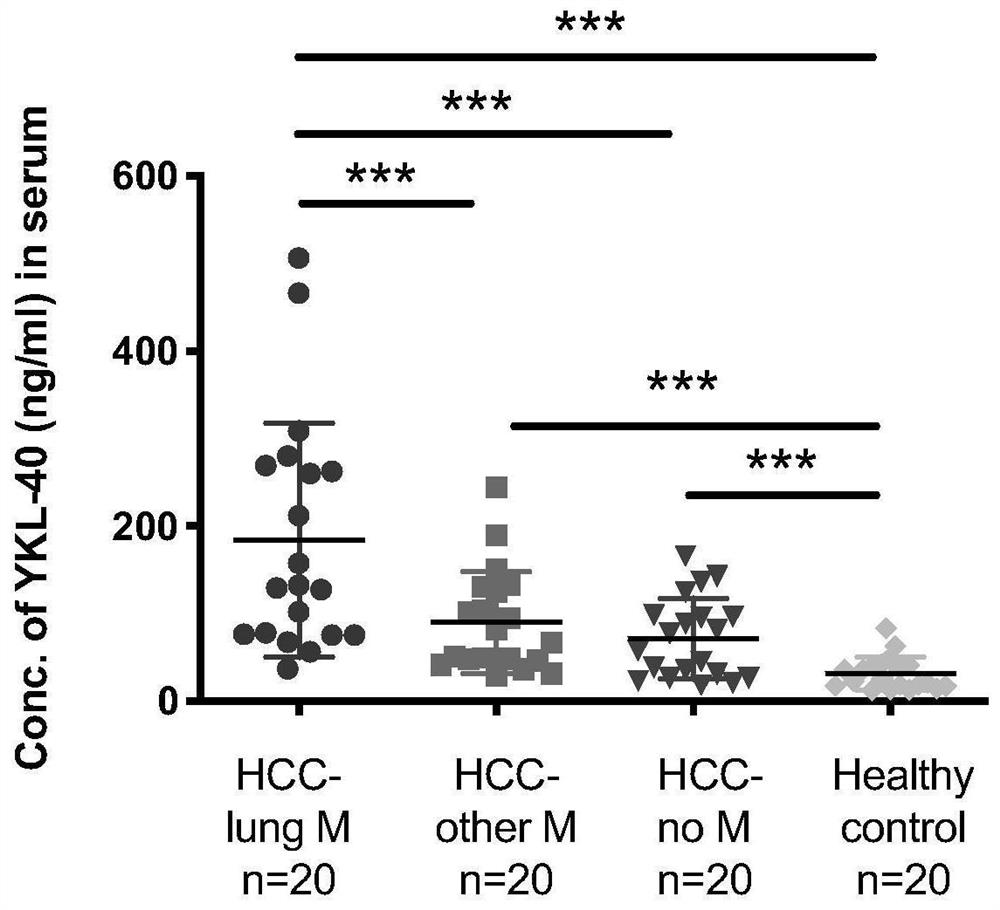
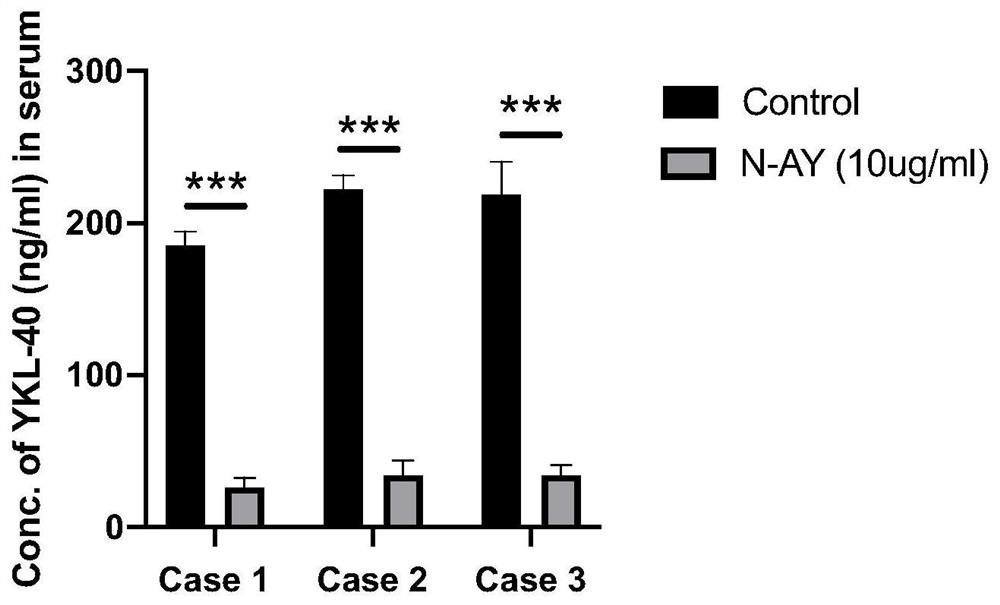
[0041] The present invention's study found that the concentration of YKL-40 in the serum of patients with liver cancer combined with lung metastasis increased significantly (attached figure 1 ). Take the serum of 3 patients with lung metastases from liver cancer, dilute the serum 1:100 with PBS in advance, add YKL-40 neutralizing monoclonal antibody at a concentration of 10ug / mL, use IgG (10ug / mL) as the control group, and incubate overnight at 4°C , ELISA detects YKL-40 concentration in serum, the results are as follows image 3 As shown, the anti-human YKL-40 neutralizing monoclonal antibody can bind YKL-40 and reduce the detectable concentration of YKL-40 in the patient's serum t...
Embodiment 3
[0052] Embodiment 3: monoclonal antibody sequencing
[0053] The YKL-40 neutralizing monoclonal antibody obtained by screening was sequenced to obtain its full-length sequence. The specific process was as follows: 1) using the TRIZol method to extract RNA from the hybridoma cell line of the monoclonal antibody;
[0054] 2) Using SMART Race technology to reverse transcribe RNA into cDNA;
[0055] 3) PCR amplification to obtain the full length of heavy chain and light chain;
[0056] 4) Use ligase to connect the target fragment to the vector, and transform the ligated product into E. coli competent cells, and then pick a single clone for sequencing;
[0057] 5) Analyze and annotate the sequencing results.
[0058] 6) The sequence information (base and amino acid) of the light and heavy chain variable regions of the monoclonal antibody is as follows:
[0059] The heavy chain variable region base sequence, as shown in SEQ ID NO: 3;
[0060] The base sequence of the light chain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com