Iron oxide powder, composition, ceramics, iron oxide powder precursor, method for producing iron oxide powder precursor, and method for producing iron oxide powder
A technology of iron oxide powder and manufacturing method, which is applied in the direction of iron oxide/iron hydroxide, chemical instruments and methods, iron oxide, etc., which can solve the problems of fading and lack of chroma in color, and achieve the effect of high heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~8、 comparative example 1~3
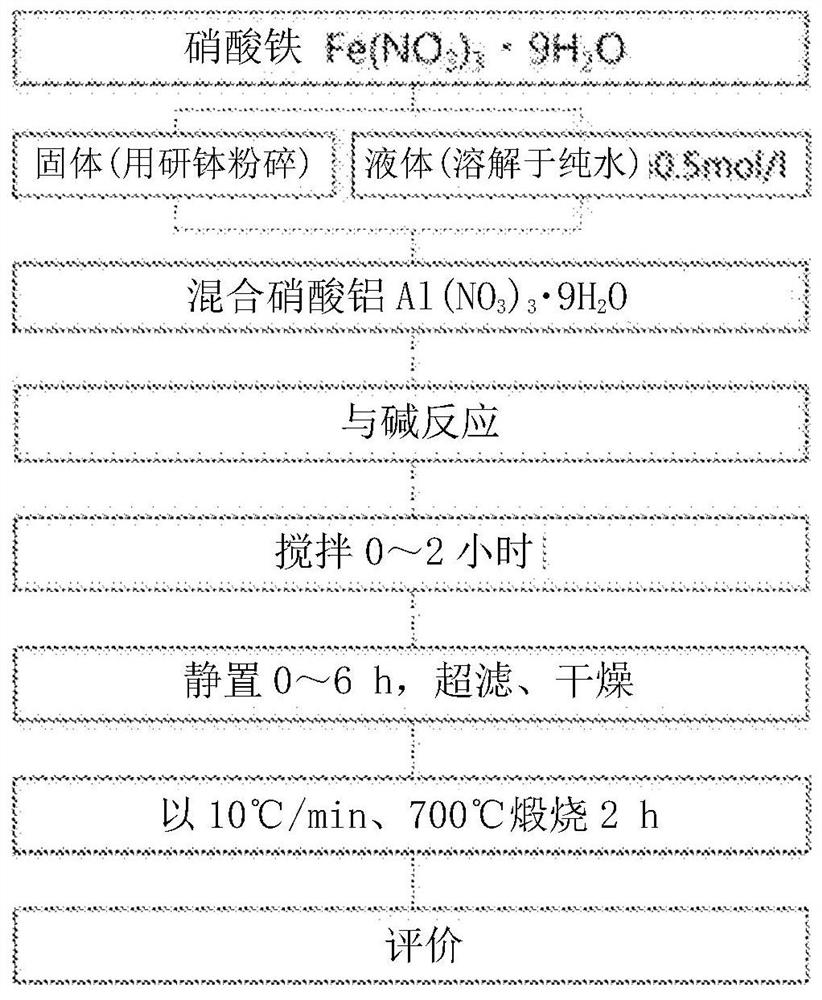
[0152] according to figure 1 According to the flow chart, iron oxide powder is obtained.
[0153] First, add 0.5mol·dm to 100ml -3 Aluminum nitrate nonahydrate is added to the ferric nitrate aqueous solution according to the condition that the value of x=Al / (Al+Fe) reaches more than 0 and less than 0.8 (that is, the content of aluminum is more than 10 mol% and less than 80 mol%), as the starting solution .
[0154] Ammonium bicarbonate in an amount 12 times the molar amount (0.6 mol) of the metal ion was gradually added to the aqueous solution with stirring.
[0155] Then, it was stirred for 0 to 2 hours and left to stand for 0 to 6 hours. The resulting suspension was suction-washed with 1 L or more of pure water, replaced with an appropriate amount of ethanol, and dried in a vacuum to obtain an iron oxide precursor. All the obtained iron oxide powder precursors were brown in color.
[0156] The iron oxide powder precursor obtained in Examples is a porous structure with a...
Embodiment 7、8
[0194] In Examples 7 and 8, the precursor samples before firing were treated at a high temperature of 1200° C. for 2 hours, and evaluated according to the above-mentioned evaluation criteria. The results are shown in Table 1.
[0195] Regarding heat resistance, referring to Table 1, it can be seen that in Examples 4 to 6, even when exposed to a high temperature of 1100°C, the chroma is maintained at the same level as that of commercially available products, and it is also known that the red color can be maintained up to 1300°C by naked eye observation. In addition, in Examples 7 and 8, even though they were heated at a high temperature of 1200° C., the chroma of the obtained iron oxide powder was also good.
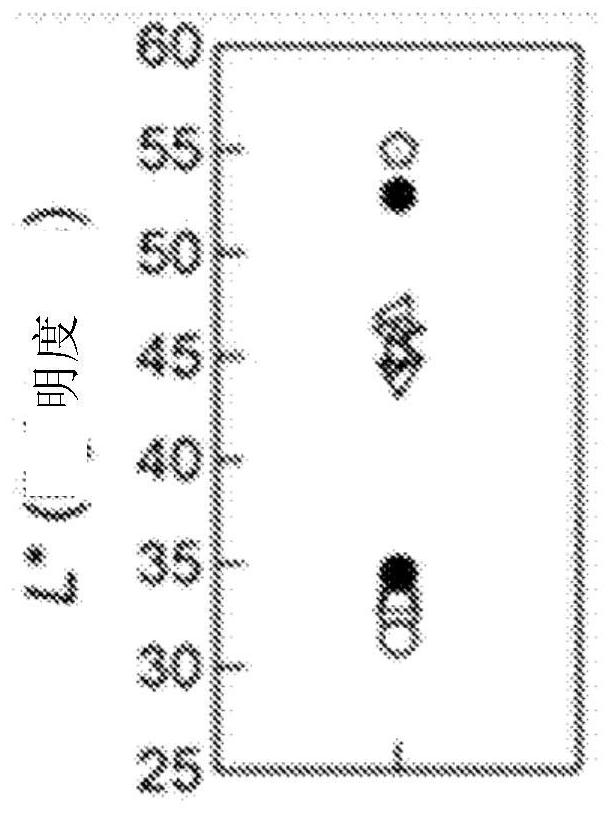
[0196] Additionally, if Figure 6 As shown, if referring to the chromaticity change when the commercial product and the powder sample of x=0.3 are heat-treated at high temperature, compared with the 1000°C reheated sample of the commercial product, the sample of the pres...
Embodiment 9-1~9-4、 comparative example 4-1~4-2
[0199] Iron oxide powder was prepared in the same manner as in Example 4 except that the concentration of the ferric nitrate aqueous solution was prepared as the value shown in Table 2. In addition, about the evaluation of heat resistance, it carried out similarly to said .
[0200] Wherein, embodiment 9-3 is identical with aforesaid embodiment 4.
[0201] [Table 2]
[0202]
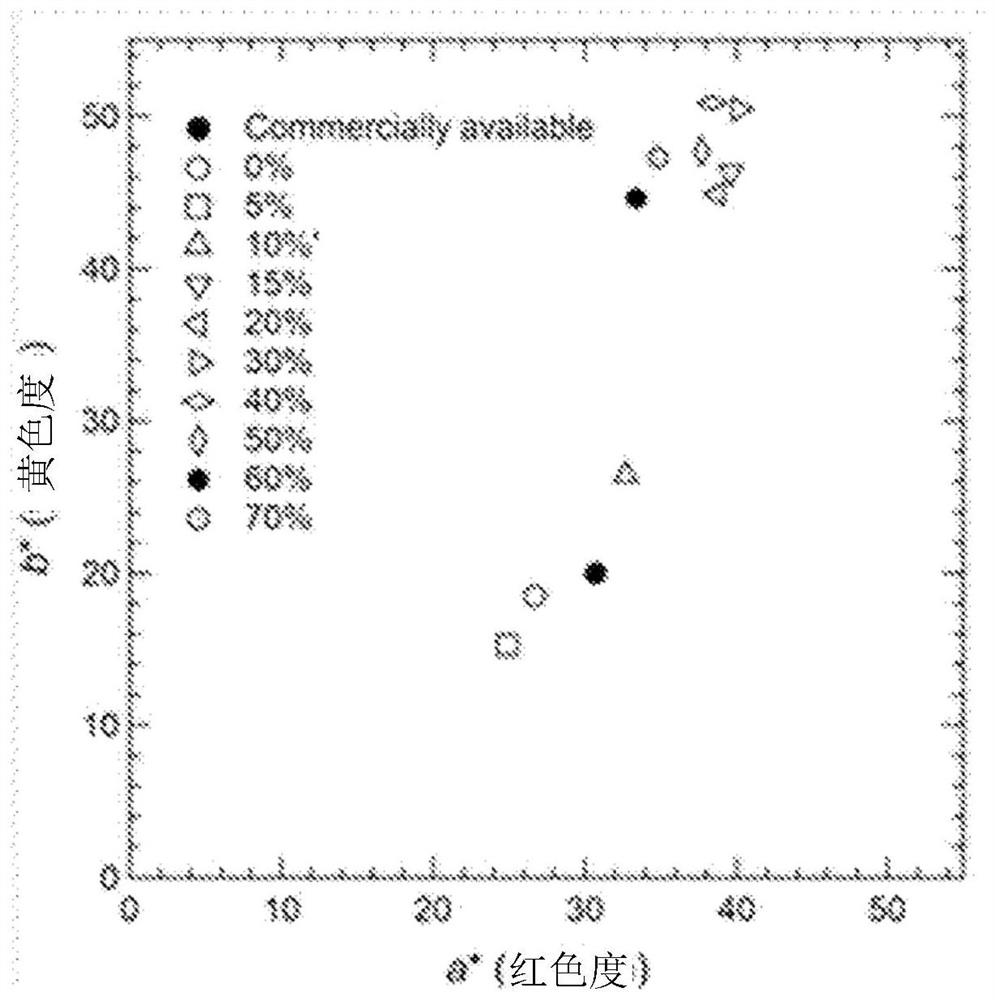
[0203] It can be seen from Table 2 that, compared with the comparative example, the iron oxide powder obtained in the examples has higher chroma and better heat resistance. In particular, the concentration of the ferric nitrate aqueous solution is 0.3mol·dm -3 ~0.7mol·dm -3 In Examples 9-2 to 9-4 prepared within the range of , iron oxide powders with extremely high chroma and high heat resistance were obtained. In addition, if Figure 7 0.3mol·dm of extremely high chroma -3 (Also denoted as "0.3M")~0.7mol·dm -3 (also referred to as "0.7M"), it was confirmed that the particle form of the iron ox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com