Flavone 3 beta-hydroxylase reductase coenzyme mutant and application thereof
A technology of hydroxylase reductase and mutants, applied in the field of genetic engineering, can solve the problem of low yield of eriodictyol, and achieve the effect of improving catalytic and production performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
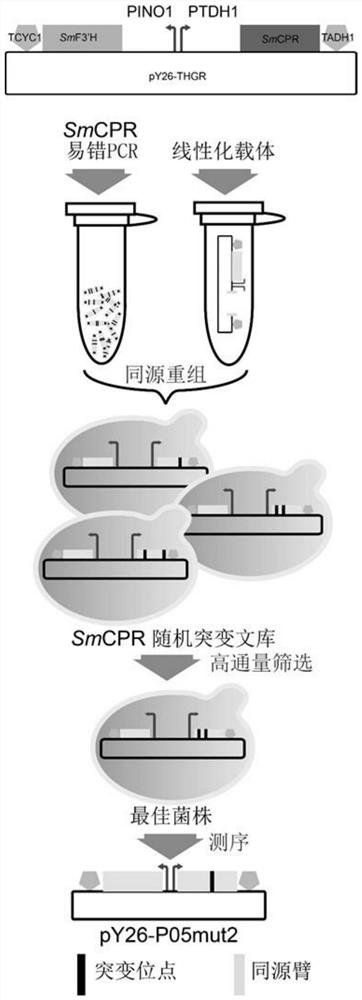
[0040] Example 1: Directed Evolution of SmCPR
[0041] Starting with plasmid pY26-P05 (pY26-P INO1 -SmF3′H-P TDH1 -SmCPR) as a template, and directed evolution of SmCPR was performed using the error-prone PCR kit GeneMorph II EZClone (Agilent, CA, US). Primers SmCPRm-F and SmCPRm-R were used to amplify and randomly mutate SmCPR, while primers 9.5k-F / 9.5k-R were used to amplify the vector backbone from plasmid pY26-P05. The PCR product was purified and recovered by precipitation. The randomly mutated SmCPR sequence shares approximately 40 bp homology arms with the linearized vector backbone DNA fragment for homologous recombination.
[0042] Mix the mutated SmCPR and the linearized carrier backbone to 50 μL (2:1, mol / mol, about 2-3 μg in total), and use the high-efficiency transformation method of Saccharomyces cerevisiae (Gietz, R.D. and R.A. Woods, Transformation of yeast bylithium acetate / single-stranded carrier DNA / polyethylene glycolmethod. Methods Enzymol, 2002. 350:...
Embodiment 2
[0044] Embodiment 2: Screening and application of mutants
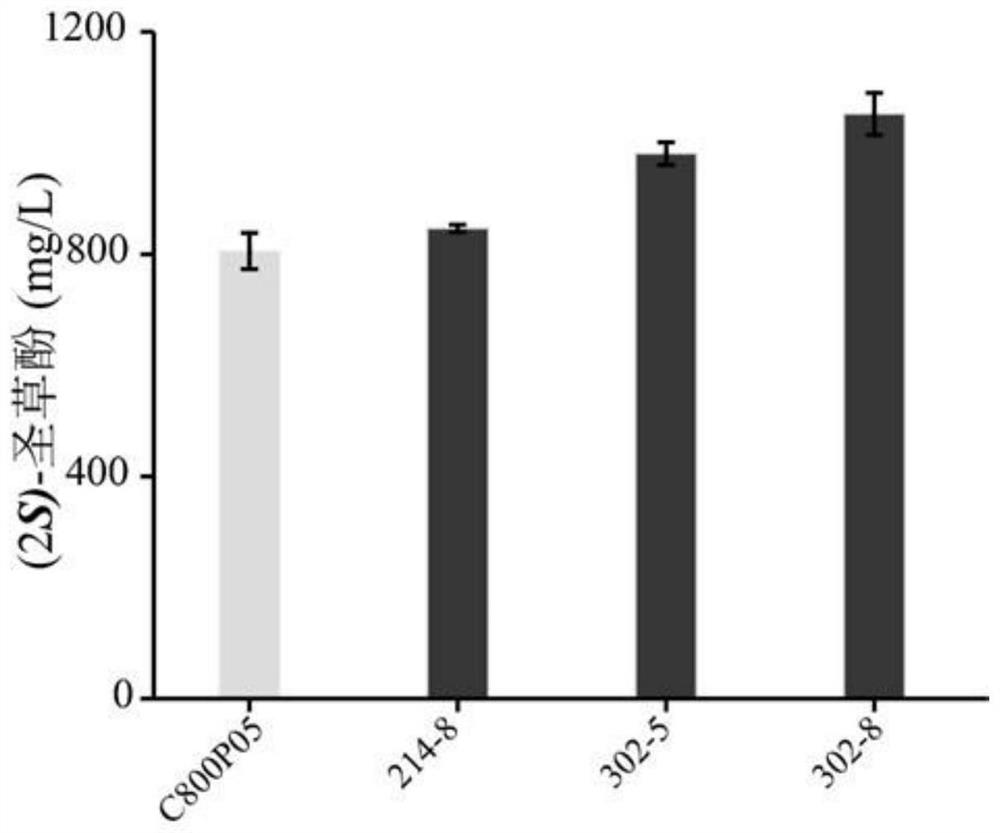
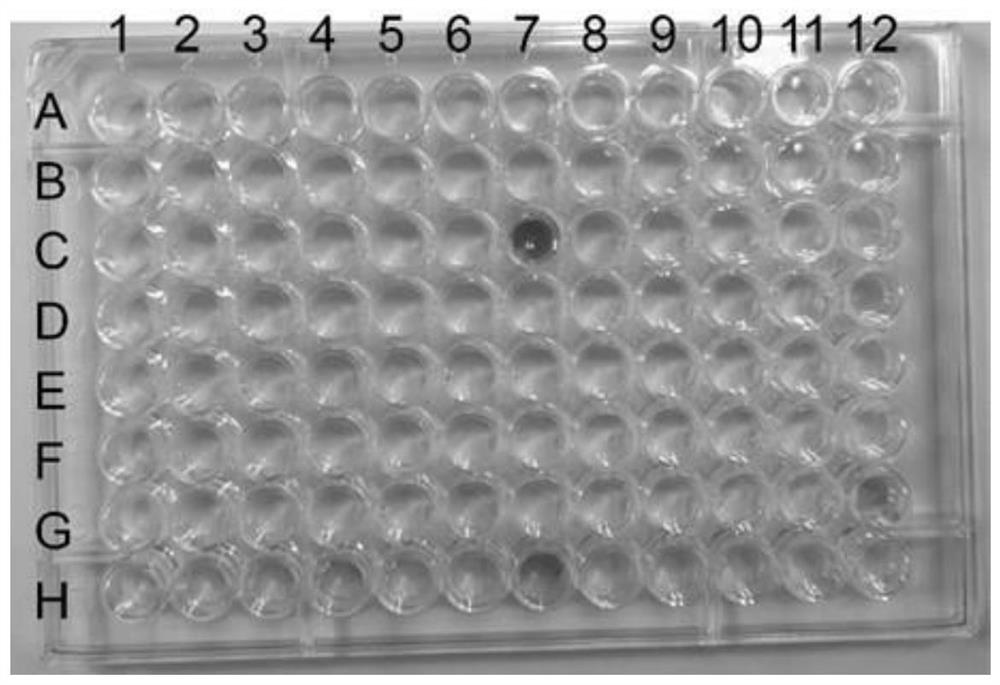
[0045] Randomly select 10,000 to 20,000 single colonies from the mutant sub-library for high-throughput screening. The obtained high-yielding strains are re-screened in shake flasks, and the strains with increased yields are determined to be sequenced to detect mutation sites. Finally, the best SmCPR was fermented at the level of 250mL shake flask to detect the yield of eriodictyol.
[0046] For the strains in the directed evolution library, use a 48-deep-well plate for cultivation: use the automatic colony-picking instrument QPix420 to automatically inoculate the colonies on the plate into a 48-deep-well plate. Add 1.5mL YNB liquid medium to each well, and the medium contains a final concentration of 250mg·L -1 of naringenin. The deep-well plate was transferred to a well-plate shaker (Zhichu, Shanghai, China), and cultured at 30° C. and 220 rpm for 48 hours. Place the deep-well plate on the table for 2 hours to pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com