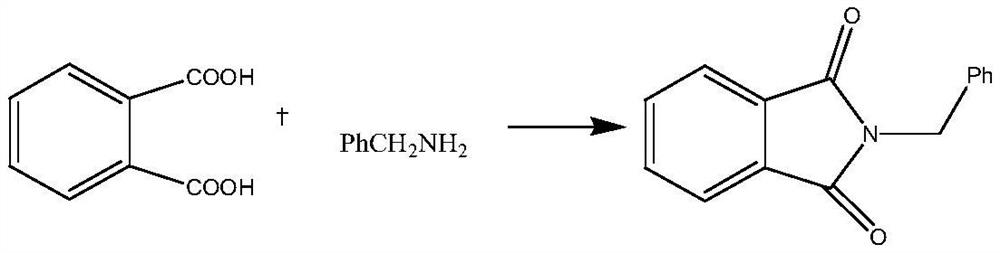
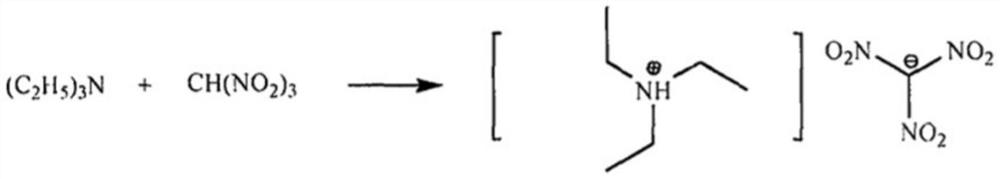
Preparation method of N-benzyl phthalimide
A technology of benzyl phthalimide and phthalic acid, which is applied in the field of preparation of N-benzyl phthalimide, can solve the problem that the reaction raw materials are not easy to mix uniformly, heat cannot be removed quickly, It is unfavorable for industrialized production and other issues, and achieves the effect of being conducive to environmental protection, simple post-processing method, and conducive to industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of triethylamine / trinitromethane eutectic catalyst:
[0025] Add 100mL of ethanol to the reactor, then add 20.2g of triethylamine and 30.2g of trinitromethane, stir and mix evenly, then stir at normal temperature and pressure for 2 hours, and distill off the solvent ethanol under reduced pressure to obtain 48.4g of yellow liquid , the yield was 96%.
Embodiment 2
[0027] Preparation of N-benzylphthalimide:
[0028] Add 100mL N,N-dimethylformamide (DMF) into the reactor, then add phthalic acid (16.3g, 0.1mol), stir and mix at room temperature, then add benzylamine (16.1g, 0.15mol) and triethylamine / trinitromethane eutectic catalyst (3g), the addition was completed, and the reaction was continued for 3 hours at room temperature and normal pressure. After the reaction, add 100mL of toluene, cool to 0-5°C, filter to obtain the target compound N-benzylphthalimide, then recrystallize with ethanol as a solvent, collect the solid by filtration, and dry it in vacuum to obtain Refined N-benzylphthalimide white needle-like solid 20.9g, its yield is 88%, and adopts HPLC area normalization method to measure its purity to be 99.8%.
Embodiment 3
[0030] Preparation of N-benzylphthalimide:
[0031] Add 100mL N,N-dimethylformamide (DMF) into the reactor, then add phthalic acid (16.5g, 0.15mol), stir and mix at room temperature, then add benzylamine (21.5g , 0.2mol) and triethylamine / trinitromethane eutectic catalyst (2g), the addition was completed, and the reaction was continued for 2 hours at room temperature and normal pressure. After the reaction, add 100mL of toluene, cool to 0-5°C, filter to obtain the target compound N-benzylphthalimide, then recrystallize with ethanol as a solvent, collect the solid by filtration, and dry it in vacuum to obtain Refined N-benzylphthalimide white needle-like solid 20.1g, its yield is 85%, and its purity measured by HPLC area normalization method is 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com