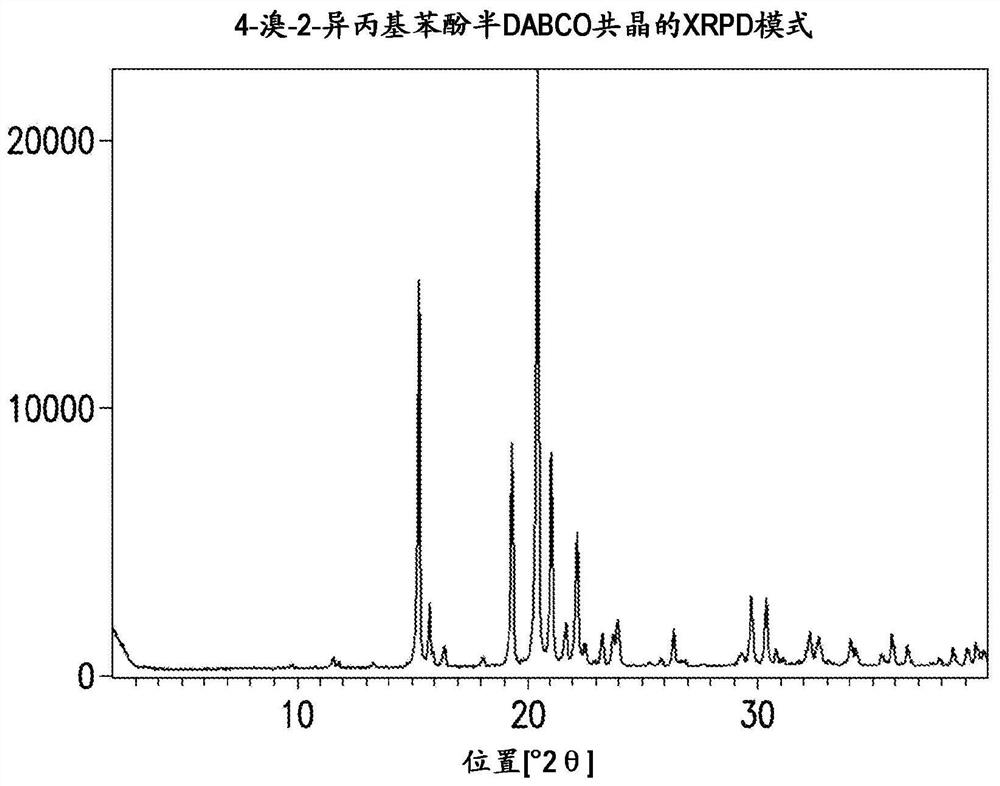
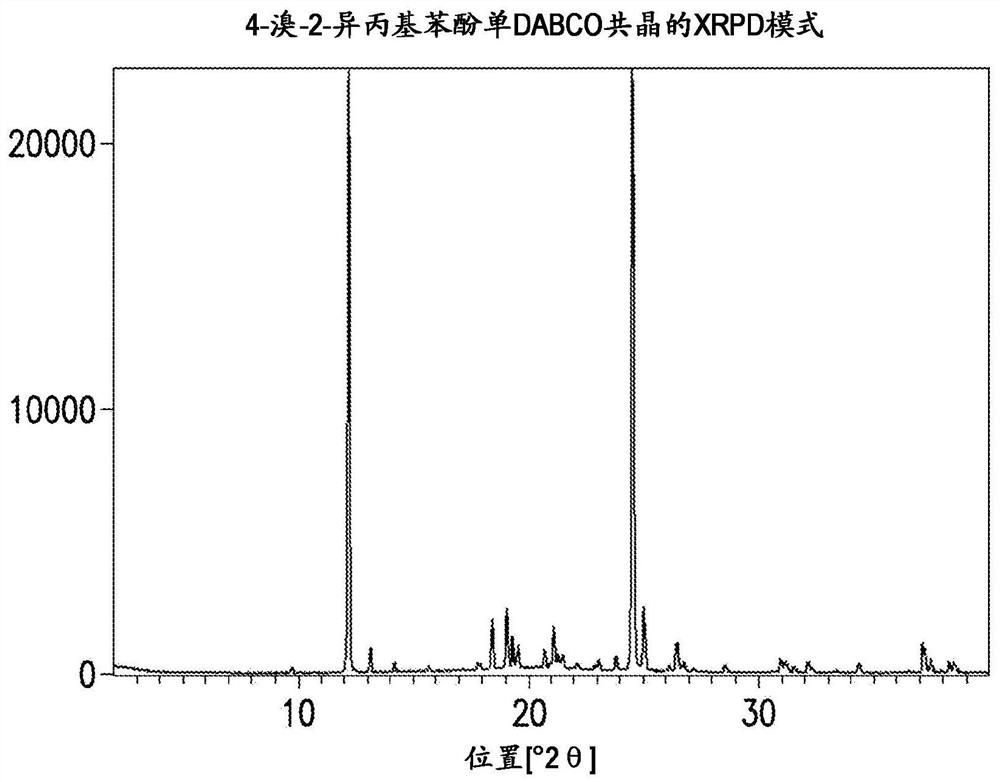
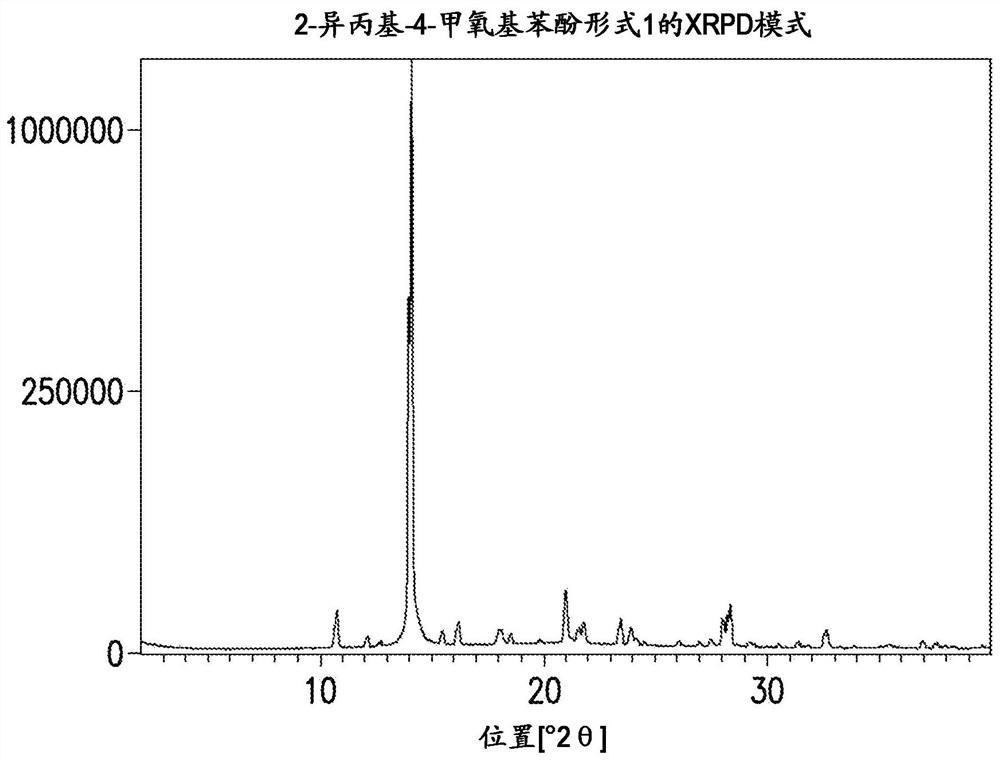
Novel process for synthesis of phenoxy diaminopyrimidine compound
A kind of technology of diaminopyrimidine and compound is applied in the field of preparing phenoxydiaminopyrimidine compound or its pharmaceutically acceptable salt, and can solve the problems of complex chemical process, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0091] The following examples are intended to be illustrative only and not limiting in any way. Abbreviations used herein and in the specification are conventional abbreviations in the art or below.
[0092] ℃ degrees Celsius
[0093] DABCO 1,4-Diazabicyclo[2.2.2]octane
[0094] DMA Dimethylamine
[0095] DMAP Dimethylaminopyridine
[0096] DMF N,N-Dimethylformamide
[0097] DMI 1,3-Dimethyl-2-imidazolinone
[0098] EtOAc ethyl acetate
[0099] EtOH ethanol
[0100] g grams
[0101] hours
[0103] kg kilogram
[0104] L liter
[0105] LC liquid chromatography
[0106] MeOH Methanol
[0107] MSA methanesulfonic acid
[0108] MeCN acetonitrile
[0109] MEK methyl ethyl ketone
[0110] min minute
[0111] mL or ml milliliter
[0112] mole mole
[0113] N equivalent concentration (normal)
[0114] NBS N-Bromosuccinimide
[0115] nM nanomole
[0116] NMP N-methyl-2-pyrrolidone
[0117] RT or rt room temperature
[0118] sat. Sa...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap