Olaparib release related pharmaceutical composition
A composition and measuring bottle technology, applied in the pharmaceutical field, can solve problems such as slow dissolution, high impurity levels, and heavy tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Get raw material according to prescription table, preparation method comprises the steps:
[0074] (1) For olaparib, add polyethylene glycol 6000, add water to dissolve, heat at 80°C, homogenize to obtain a olaparib suspension, spray dry, and make a dry olaparib solid dispersion;
[0075] (2) Gradually add sodium lauryl sulfate, mannitol, crospovidone, talc powder, and micropowder silica gel, sieve and mix repeatedly, and directly compress into tablets to obtain olaparib tablets.
[0076] test results:
[0077] Compressibility: good
[0078] Friability: in compliance with regulations
[0079] Appearance: smooth and beautiful
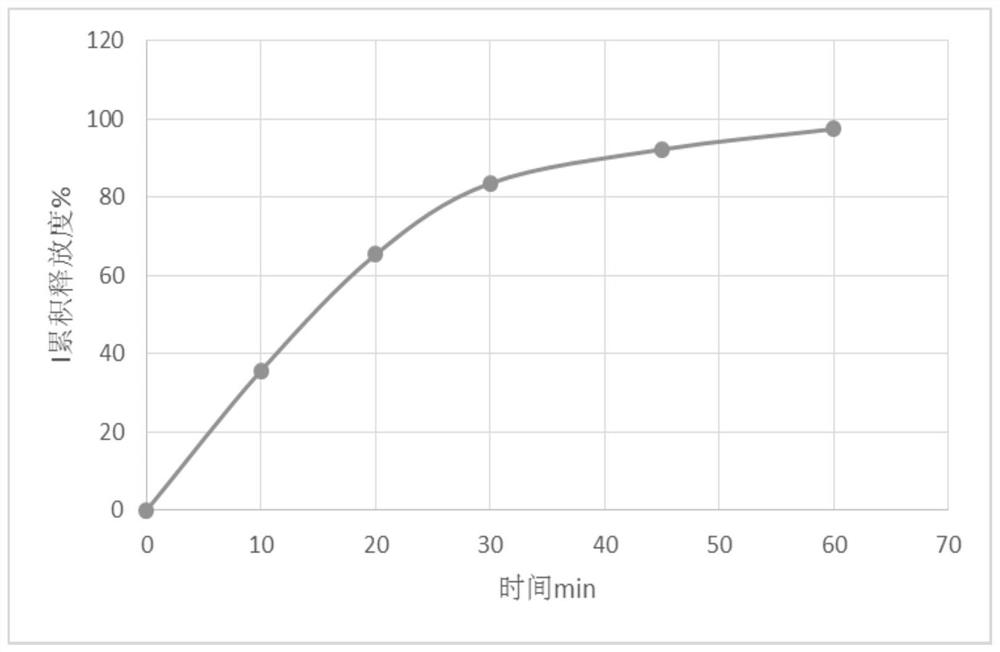
[0080] 45min average dissolution rate: 92.3%
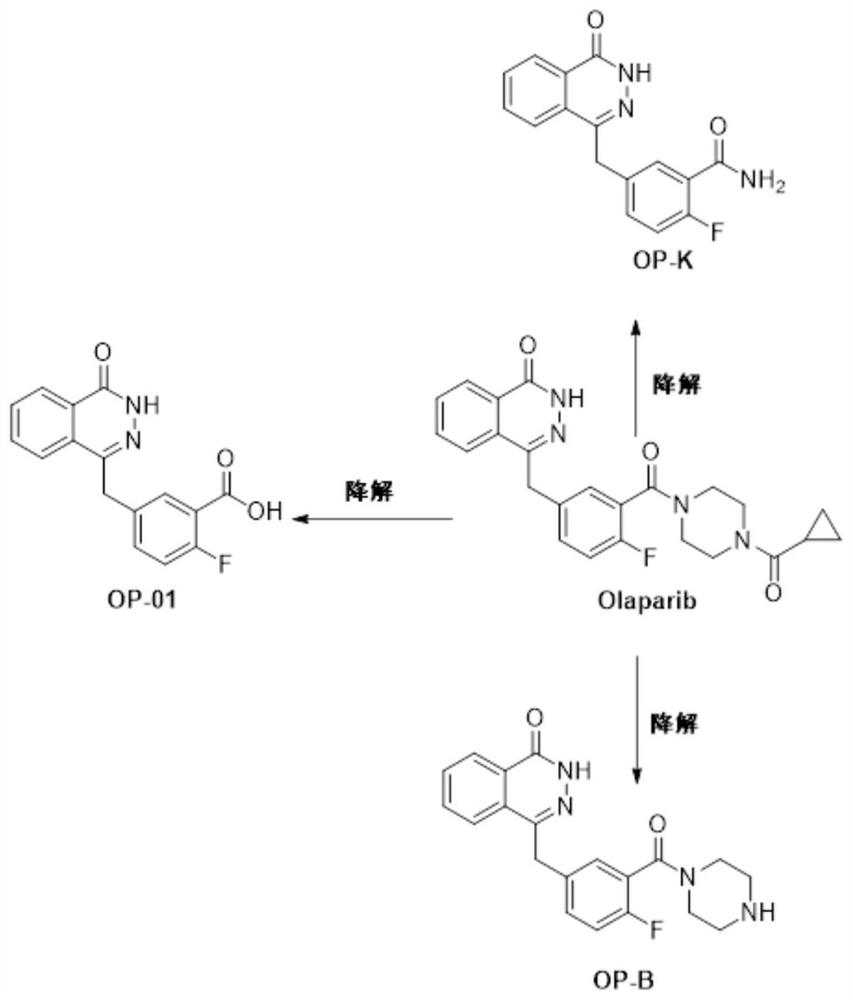
[0081] Related substance results: OP-K, OP-B, OP-01 were not detected.
Embodiment 2
[0083] Get raw material according to prescription table, preparation method comprises the steps:
[0084] (1) Take olaparib and polyethylene glycol 6000, add water to dissolve, heat at 80°C, homogenize to obtain a olaparib suspension, spray dry, and make a dry olaparib solid dispersion;
[0085] (2) Gradually add sodium lauryl sulfate, mannitol, crospovidone, mannitol, and micropowdered silica gel, sieve and mix repeatedly, and directly compress into tablets to obtain olaparib tablets.
[0086] Related substance and stripping curve determination method are the same as embodiment 1
[0087] test results:
[0088] Compressibility: good
[0089] Friability: in compliance with regulations
[0090] Appearance: smooth and beautiful
[0091] 45min average dissolution rate: 71.5%
[0092] Related substance results: OP-K, OP-B, OP-01 were not detected.
Embodiment 3
[0094] Get raw material according to prescription table, preparation method comprises the steps:
[0095] (1) Take olaparib, add polyethylene glycol 6000, stir to mix evenly, heat at 80°C, homogenize to obtain olaparib suspension, spray dry to make dry olaparib solid Dispersions;
[0096] (2) Gradually add sodium lauryl sulfate, mannitol, crospovidone, mannitol, micronized silica gel, talcum powder, sieve and mix repeatedly, and directly compress into tablets to obtain olaparib tablets.
[0097] Related substance and stripping curve determination method are the same as embodiment 1
[0098] test results:
[0099] Compressibility: good
[0100] Friability: in compliance with regulations
[0101] Appearance: smooth and beautiful
[0102] 45min average dissolution rate: 79.3%
[0103] Related substance results: OP-K, OP-B, OP-01 were not detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com