Anthraquinone compound and preparation method of anthraquinone compound from ranunculus davidii
A compound, the technology of anthraquinones, applied in the field of preparation of anthraquinones, can solve the problem of not separating out anthraquinones
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] In a second aspect, the present invention provides a method for preparing the anthraquinone compound from Buttercup thorn, comprising the following steps:
[0037] The total extract is obtained after pulverizing, extracting, and concentrating the extract;
[0038] The total extract was separated by silica gel column chromatography, and chloroform-methanol system gradient elution was used to obtain 8 components in sequence;
[0039] The fifth component is eluted with a gradient of chloroform-methanol to obtain the compound of formula (I) and compound of formula (II).
[0040] In some embodiments, the gradient elution procedure for the fifth component using the chloroform-methanol system is: 100:5-20, v / v.
[0041] In some embodiments, the compound β-glucopyranoside is also obtained after the fifth component is eluted with a chloroform-methanol system gradient.
[0042] In some embodiments, the crushed Buttercup thorn has a particle size of 20-40 mesh.
[0043] In some...
Embodiment
[0049] experiment apparatus
[0050] Nicolet-510P infrared spectrometer (USA), v max in cm -1 The unit is Bruker AMX-400 nuclear magnetic resonance instrument, TMS is the internal standard, the chemical shift is expressed in δ, and the coupling constant is expressed in J.
[0051] Extraction and Separation Methods
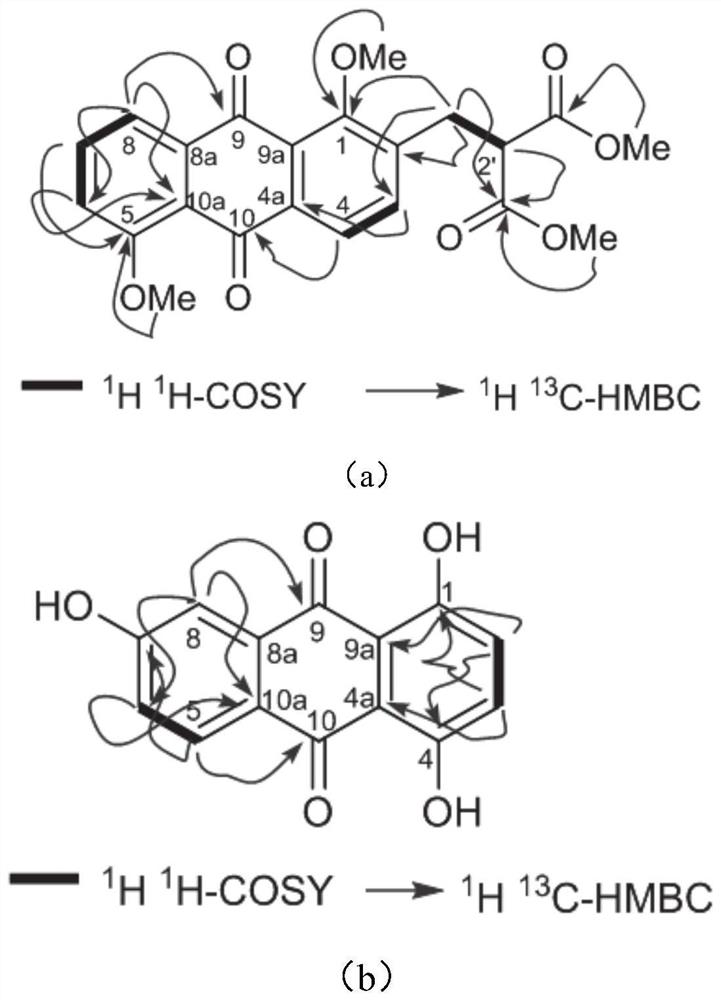
[0052]3.4kg of ranunculus thorns were crushed, leached with 10L ethanol at room temperature, the filtrate was filtered, and concentrated under reduced pressure to obtain 6.5g of the total extract. The total extract was separated by silica gel column chromatography, and the chloroform-methanol system was used for gradient elution (100:5~100:20, v / v). This time, (100:5, v / v), (100: 10, v / v), (100:15, v / v) and (100:20, v / v) were eluted to obtain 8 fractions (F1-8), and fraction F5 (155 mg) was treated with chloroform-methanol Gradient elution of the system (100:10, v / v) and (100:15, v / v) gave compound muracatane A (10.4 mg), compound muracatane B (5.6 mg) and comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com