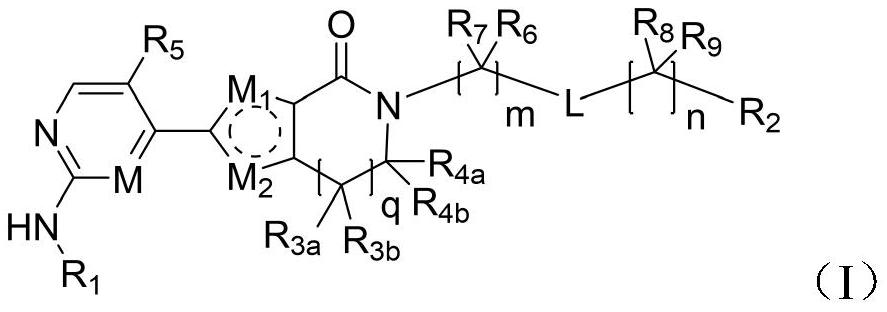
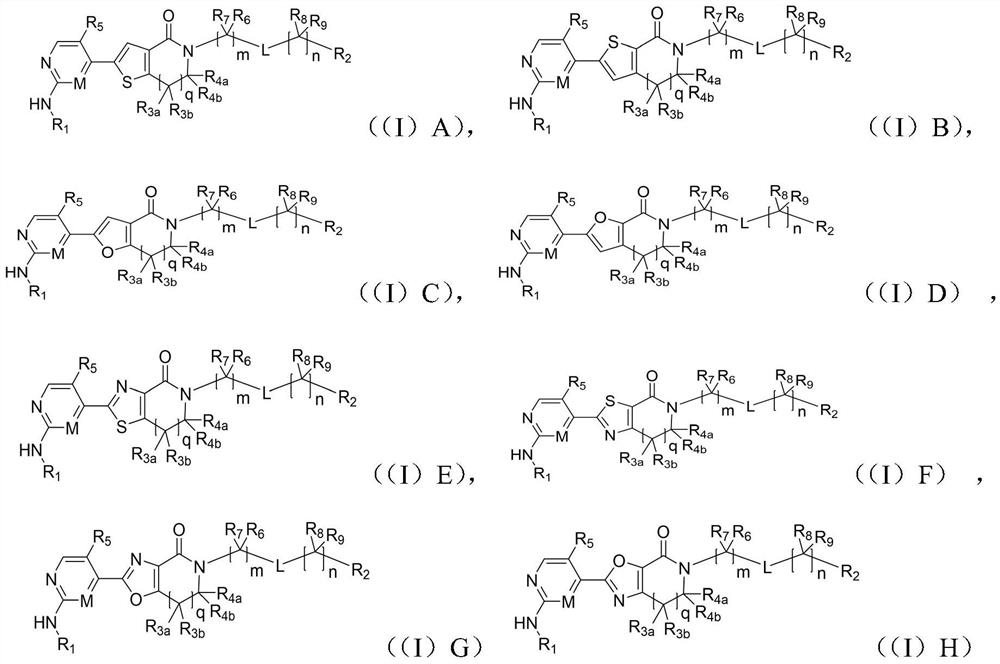
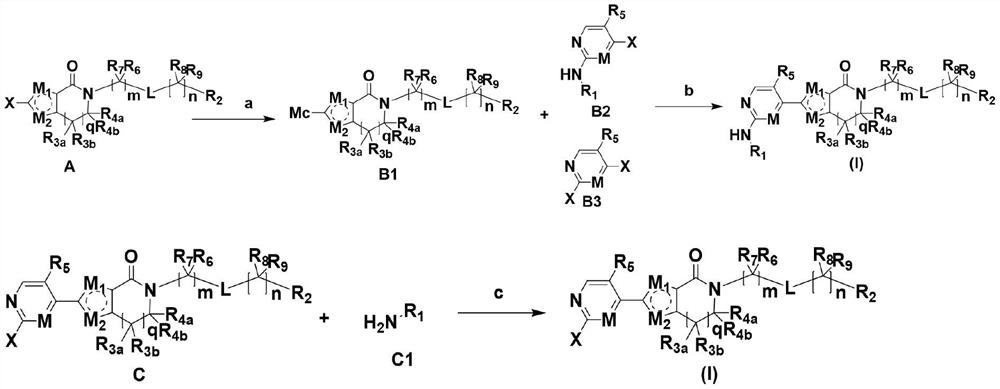
A class of aromatic heterocyclic lactam compounds, preparation method and use
A compound and heterocycloalkyl technology, applied in the field of medicinal chemistry, can solve the problems of single structure, easy drug resistance, low activity, etc., and achieve the effects of good ERK kinase inhibitory activity, novel structure, and excellent cell proliferation inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0196] Example 1: (R)-N-((S)-1-(3-chlorophenyl)-2-hydroxyethyl)-2-(2-(2-((1-methyl-1H-pyridine Azol-5-yl)amino)pyridin-4-yl)-4-oxo-4,6-dihydro-5H-thien[2,3-c]pyrrol-5-yl)propionamide
[0197]
[0198] The first step: (4-bromopyridin-2-yl)(1-methyl-1H-pyrazol-5-yl) tert-butyl carbamate (150mg, 0.42mmol), pinacol borate (119mg, 0.47mmol), potassium acetate (123mg, 1.26mmol) and 1,1'-bisdiphenylphosphinoferrocenepalladium dichloride (Pd(dppf)Cl 2 ) (61mg, 0.084mmol) was dissolved in dioxane (10mL), nitrogen was bubbled for 10 minutes, and heated to 80°C for 8 hours under nitrogen protection. The reaction solution was cooled to room temperature, filtered, and concentrated under reduced pressure to obtain (1-methyl-1H-pyrazol-5-yl)(4-(4,4,5,5-tetramethyl-1,3,2- Dioxin-2-yl)pyridin-2-yl)tert-butyl carbamate (yellow oil) was directly used in the next reaction. LC-MS: ESI [M+H]+=401.2; 1 H-NMR(DMSO-d6,400MHz)δ8.34-8.35(m,1H),7.92(s,1H),7.88(s,1H),7.38(d,J=2.0Hz,1H),6.14(d , J=...
Embodiment 2
[0203] Example 2: (R)-N-((S)-1-(3-chlorophenyl)-2-hydroxyethyl)-2-(2-(5-methyl-2-((1-methyl Base-1H-pyrazol-5-yl)amino)pyrimidin-4-yl)-4-oxo-4,6-dihydro-5H-thieno[2,3-c]pyrrol-5-yl)propane Amide
[0204]
[0205] The first step: tert-butyl (R)-2-(4-oxo4,6-dihydro-5H-thieno[2,3-c]pyrrol-5-yl)propionate (1.0g, 3.8 mmol), bispinacol borate (B 2 pin 2 ) (531 mg, 2.1 mmol), 4,4-di-tert-butylbipyridine (dtbpy) (20 mg, 0.076 mmol) and 1,5-cyclooctadiene methoxyiridium [Ir (cod) OMe] 2 ( 25mg, 0.038mmol) was dissolved in tetrahydrofuran (THF) (20mL), replaced with nitrogen for 10 minutes, and heated to 80°C under the protection of nitrogen to react overnight. The reaction solution was cooled to room temperature, filtered, and concentrated under reduced pressure to obtain (R)-2-(4-oxo-2-(4,4,5,5-tetramethyl-1,3,2-dioxin-2 -yl)-4,6-dihydro-5H-thieno[2,3-c]pyrrol-5-yl)propanoic acid tert-butyl ester (1.4g, red oily substance), directly used in the next reaction. LC-MS:ESI[M+H] ...
Embodiment 3
[0210] Example 3: (2R)-N-(2-hydroxyl-1-(m-toluene)ethyl)-2-(2-(5-methyl-2-((1-methyl-1H-pyrazole -5-yl)amino)pyridin-4-yl)-4-oxo-4,6-dihydro-5H-thiophene[2,3-c]pyrrol-5-yl)propionamide
[0211] Example 3 was synthesized by the same method as Example 1, LC-MS: ESI (M+H) 530.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com