Pharmaceutical composition for prevention or treatment of kidney damage
A composition and technology for acute kidney injury, applied in the direction of drug combination, drug delivery, medical preparations containing active ingredients, etc., can solve problems affecting organ damage and protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
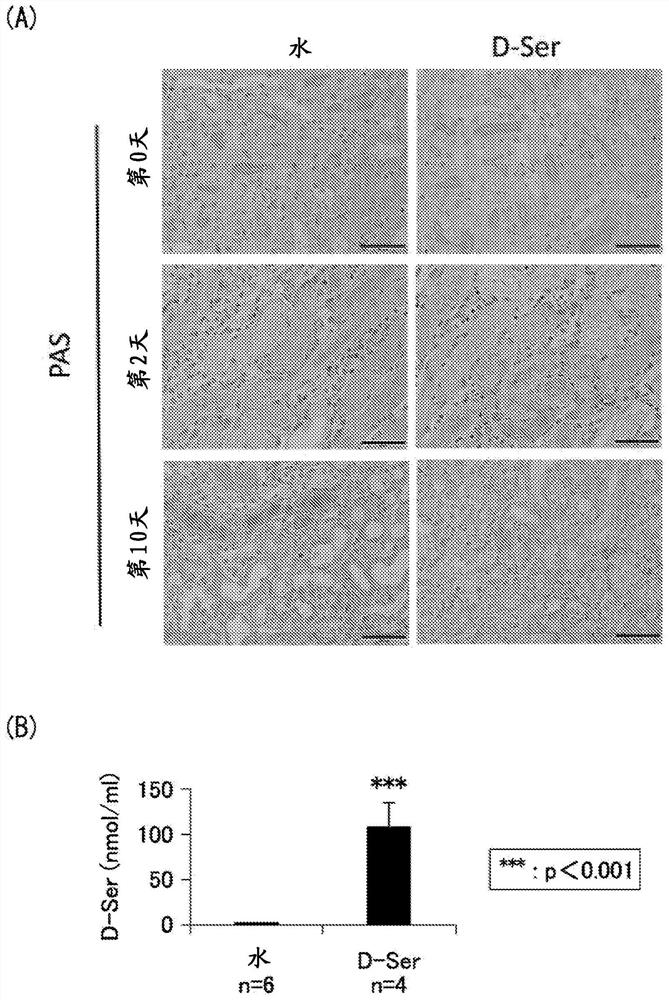
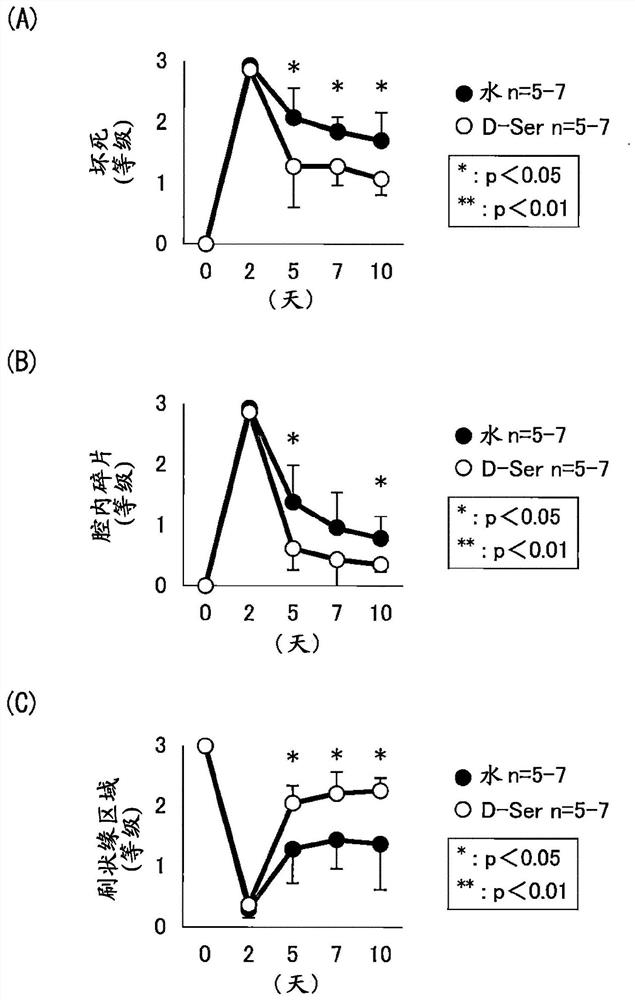
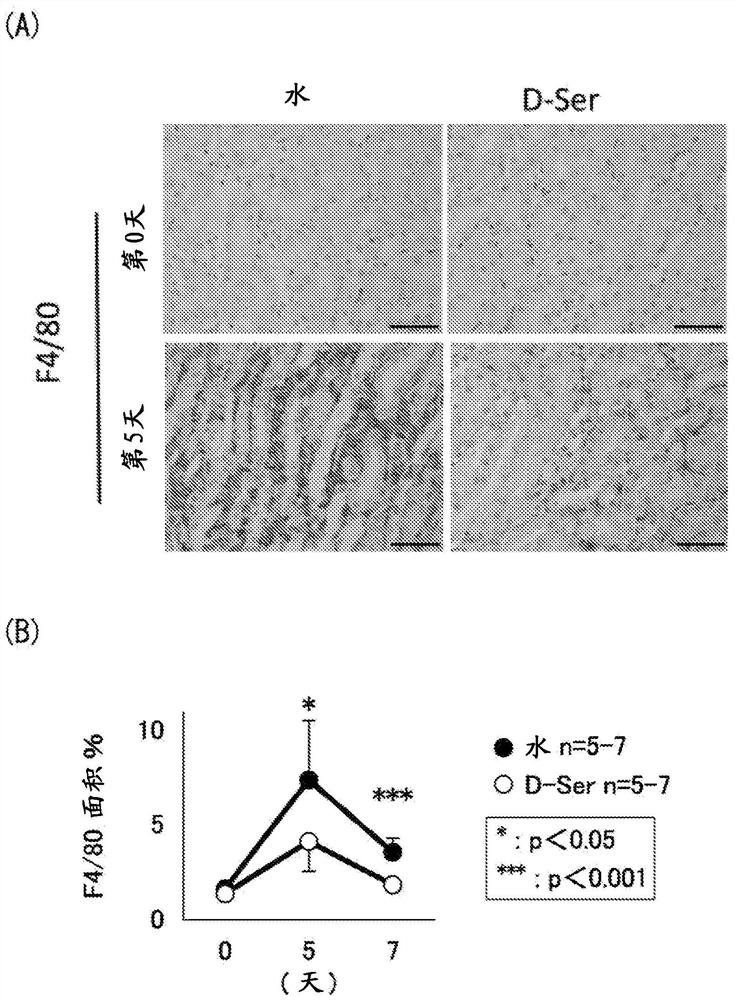
[0071] Example 1: Induction of renal injury by ischemia-reperfusion
[0072] 1. Materials and Methods
[0073] (1) Research ethics
[0074] All experiments were performed in accordance with facility guidelines and were approved by the facility's Animal Experimentation Committee.
[0075] (2) Material
[0076] Amino acid enantiomers and HPLC grade acetonitrile were purchased from Nakalai Tesque (Kyoto). HPLC grade methanol, trifluoroacetic acid, boric acid, etc. were purchased from Wako Pure Chemical Industries (Osaka). Water was purified using a Milli-Q gradient A10 system.
[0077] (3) animals
[0078] Animals were raised in an SPF environment under conditions of alternating light and dark every 12 hours, with free access to water and feed. C57BL / 6J mice were purchased from Japan Clear (Osaka).
[0079] (4) Renal ischemia-reperfusion treatment
[0080] Male mice aged 12-16 weeks were subjected to renal ischemia-reperfusion (hereinafter also referred to as "IRI") tre...
Embodiment 2
[0087] Example 2: Hypoxic stress on renal tubular epithelial cells (TEC)
[0088] mProx24 cells as mouse renal tubular epithelial cells were provided by Sugaya (St. Marianna University School of Medicine, Tokyo). The cells were cultured in DMEM medium supplemented with 5% fetal bovine serum (FBS) and 1% penicillin and streptomycin. The cultured cells were diluted with 1.0×10 DMEM medium supplemented with 1% FBS 6 Cells / well seeding at 37°C, 5% CO 2 and 20%O 2 Incubate for 24 hours under a humidified atmosphere. In the hypoxic stress group, after 24 hours of culture, in DMEM medium supplemented with 5% FBS in 5% CO 2 and 5%O 2 Further cultured in a humidified atmosphere for 20 hours, in the non-hypoxic stress group, in DMEM medium supplemented with 5% FBS in 5% CO 2 and 20%O 2 Further cultured for 20 hours in a humidified atmosphere ( Figure 4 (A)). 1 μM, 10 μM, and 100 μM of D-serine were added as a test drug to these DMEM media, and D-serine was not contained in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com