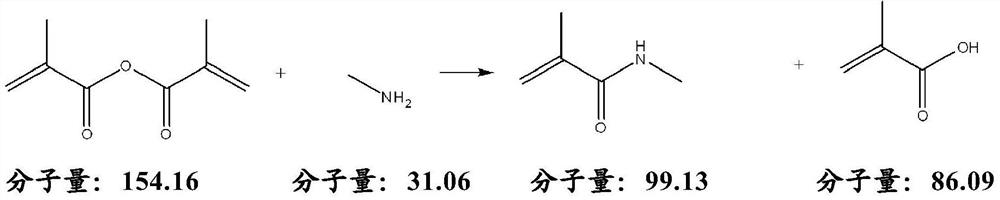
Process for preparing n-methyl(METH)acrylamide
A technology of acrylamide and methyl, applied in the field of preparing N-methacrylamide, achieves the effect of complete atom economy and low loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the preparation of N-methylmethacrylamide
[0048] Reaction equation:
[0049]
[0050] Apparatus: 2 liter autoclave with glass insert, Ni-Cr-Ni thermocouple, gas feed: metal, steel methylamine cylinder, manometer, acetone / dry ice bath
[0051] mixture:
[0052] 6.0mol methacrylic anhydride = 940.2g
[0053] 6.0mol methylamine, gas=186.4g
[0054] Theoretical yield: (=starting weight) 1126.6g
[0055] Procedure: The methacrylic anhydride was initially added to the glass insert of the autoclave and the autoclave was screwed shut. The steel methylamine cylinder was equilibrated and connected to the autoclave by a coiled VA feed tube; thus the loss in weight as methylamine was introduced could be monitored.
[0056] The introduction of methylamine was started at room temperature at a metering rate of about 4 g / 3 min. The reaction is strongly exothermic. The temperature should not exceed 40°C (35°C±5°C). The mixture was cooled with an acetone-dry ice ...
Embodiment 2
[0061] Example 2: Workup of N-methylmethacrylamide by distillation
[0062] Apparatus: 2 liter three-neck round bottom flask with boiling capillary, Pt100 temperature sensor, 30 cm mirrored column fitted with 8 x 8 Raschig rings, automatic column head (dispenser), reflux Condenser, coiled condenser, Thiele-Anschütz connection, receiver, oil bath, vacuum pump, pressure gauge
[0063] mixture:
[0064] 1122 g of N-methylmethacrylamide obtained from Example 1
[0065] 22.4mg 4-Hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (free radical) (20ppm)
[0066] 224.4mg hydroquinone monomethyl ether (200ppm)
[0067] 1122mg octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate (1000ppm)
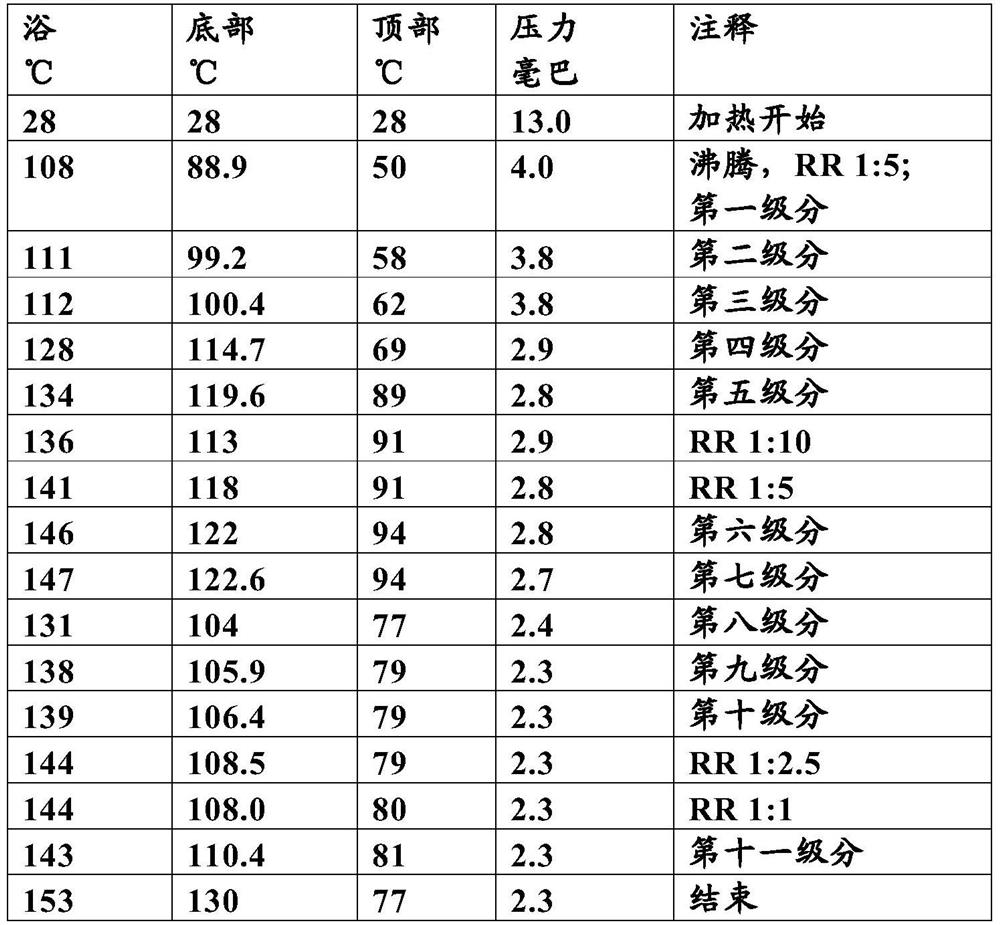
[0068] Operation process:
[0069]
[0070] RR = reflux ratio
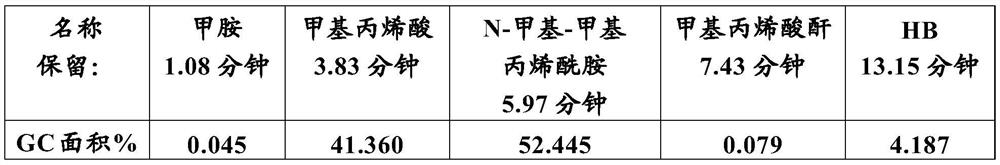
[0071] Yield and GC analysis:
[0072]
[0073] *Viscous, additional peaks: 11.257 minutes: 25.653%; 11.688 minutes: 15.458%; 12.259 minutes: 5.177% + additional smaller peaks
Embodiment 3
[0074] Embodiment 3: Preparation of N-methylmethacrylamide in solvent
[0075] Apparatus: 1 liter four-neck round bottom flask with precision glass stirrer (PTFE sleeve), Pt100 temperature sensor, gas inlet (PTFE), gas outlet of PTFE with There are wash bottles as safety bottles, reflux condensers, steel methylamine cylinders, exhaust ducts leading directly into fume hoods, compressed air as feed for added air, acetone / dry ice cooling bath
[0076] mixture:
[0077] 1.0mol methacrylic anhydride = 156.4g
[0078] 250ml methyl tert-butyl ether (MTBE)
[0079] 1.0mol methylamine, gas
[0080] Theoretical yield: 99.1g
[0081] Procedure: Add methacrylic anhydride and MTBE initially and allow to cool. Methylamine gas was introduced at about 2°C-10°C. The reaction is slightly exothermic.
[0082] Since the reaction proceeds only very slowly at this temperature, the gas is introduced more quickly and the bottom temperature can be raised up to 50°C. The introduction was termin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com