Beta-sulfonyl chiral amino compound and preparation method thereof
An amino compound and chiral technology, applied in the field of beta-sulfone-based chiral amino compounds and their preparation, to achieve the effects of shortening the reaction route, the reaction process being safe and controllable, and reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
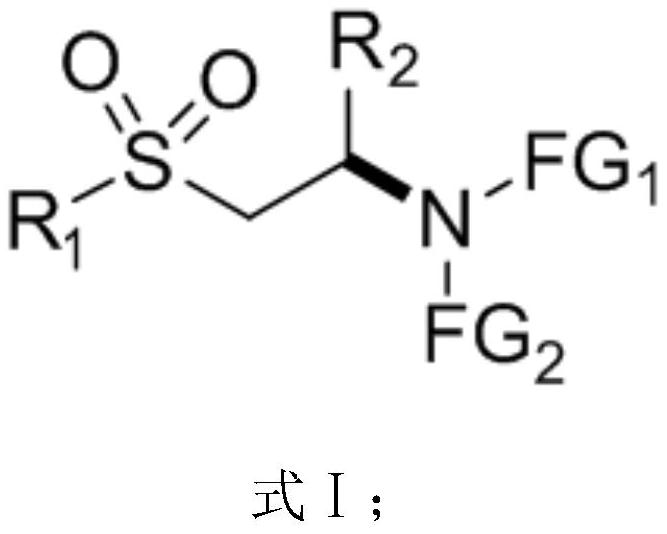
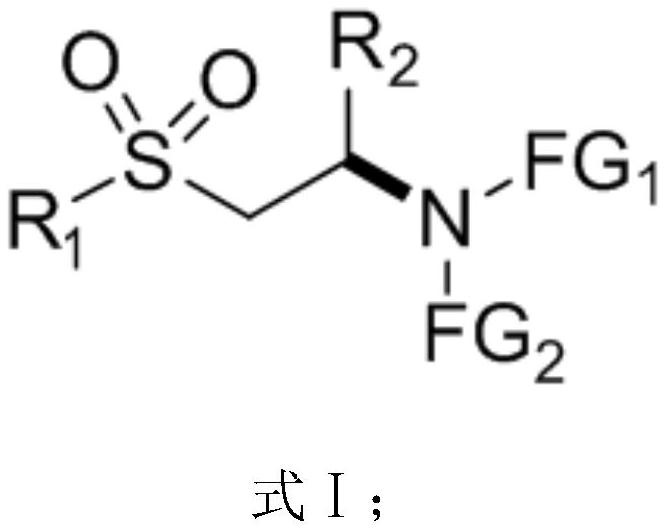
[0071] On the other hand, on the basis of the β-sulfone-based chiral amino compound described above, the embodiment of the present application also provides a preparation method of the β-sulfone-based chiral amino compound of the general formula I above, Including the following steps:
[0072] S01: Provide nucleophile compound A and conjugated sulfone compound B represented by the following structural formula:
[0073]
[0074] S02: Add the nucleophile compound A and the conjugated sulfone compound B into a reaction system containing a nitrogen heterocyclic carbene catalyst and an alkaline reagent, and perform an asymmetric Michael addition reaction to obtain a general structural formula such as formula I The β-sulfone-based chiral amino compound shown.
[0075] FG in the above-mentioned nucleophile compound A molecular structural formula 1 and FG 2 The represented group is the same as FG in the β-sulfone chiral amino compound shown in the above formula I 1 and FG 2 Th...
Embodiment 1
[0090] This embodiment provides a kind of (R)-(benzyloxy)(1,1,1-trifluoro-3-(phenylsulfonyl)propan-2-yl)carbamate tert-butyl ester and its preparation method, which Structural formula is shown in following molecular structural formula I1:
[0091]
[0092] Its preparation steps are as follows:
[0093] In a dry 10mL test tube, add mesitylene-substituted indenol-derived triazolecarbene catalyst (0.02mmol, 0.2equiv.), 0.6mL anhydrous toluene, replace with argon three times, add base (0.02mmol, 0.2eq) to react The test tube was sealed and stirred at room temperature for 30 min. The nucleophile tert-butyl carbamate (0.12mmol, 1.2eq) was slowly added to the reaction system and stirred at room temperature for 0.5 hours. The corresponding β-trifluoromethyl unsaturated sulfone compound (0.1 mmol, 1.0 equiv.) was slowly added to the reaction system, and the resulting mixture was stirred at room temperature for 12 hours. After the reaction, the reaction solution was filtered throu...
Embodiment 2
[0096] This example provides a chiral (R)-(benzyloxy)(1,1,1-trifluoro-3-(pyridin-2-ylsulfonyl)propan-2-yl)carbamate tert-butyl ester And preparation method thereof, its structural formula is shown in following molecular structural formula I8:
[0097]
[0098] Its preparation steps are as follows:
[0099] In a dry 10mL test tube, add mesitylene-substituted indenol-derived triazolecarbene catalyst (0.02mmol, 0.2equiv.), 0.6mL anhydrous toluene, replace with argon three times, add base (0.02mmol, 0.2eq) to react The test tube was sealed and stirred at room temperature for 30 min. The nucleophile tert-butyl carbamate (0.12mmol, 1.2eq) was slowly added to the reaction system and stirred at room temperature for 0.5 hours. The corresponding β-trifluoromethyl unsaturated sulfone compound (0.1 mmol, 1.0 equiv.) was slowly added to the reaction system, and the resulting mixture was stirred at room temperature for 12 hours. After the reaction, the reaction solution was filtered t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com