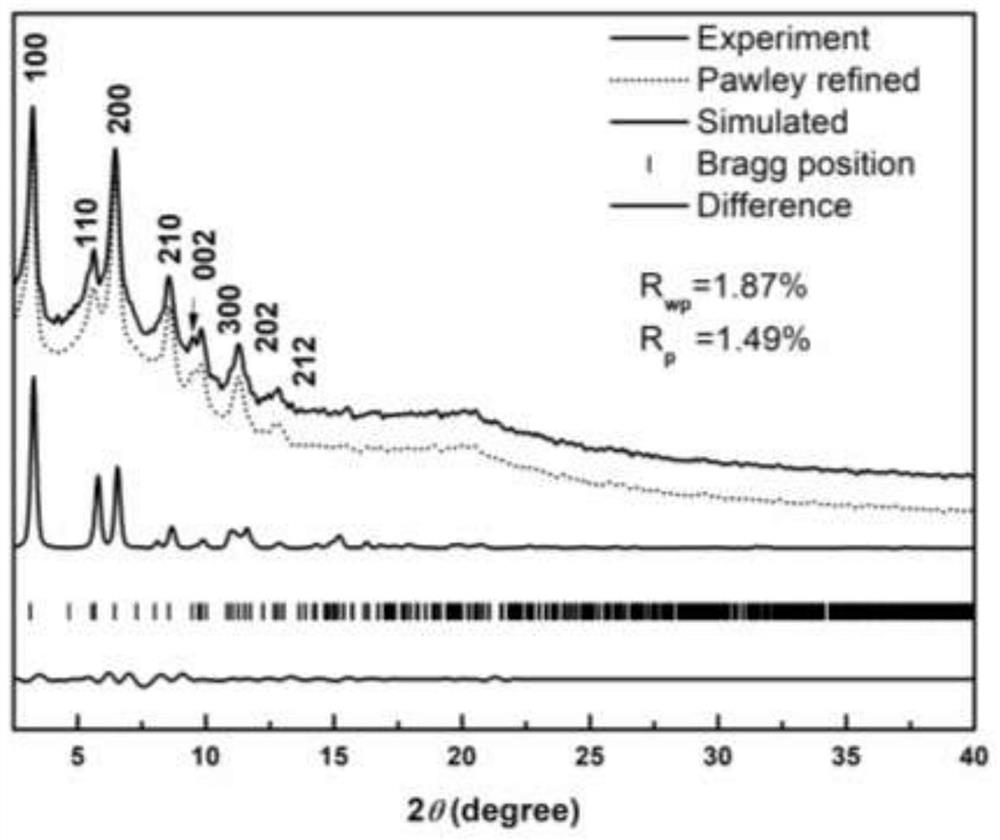
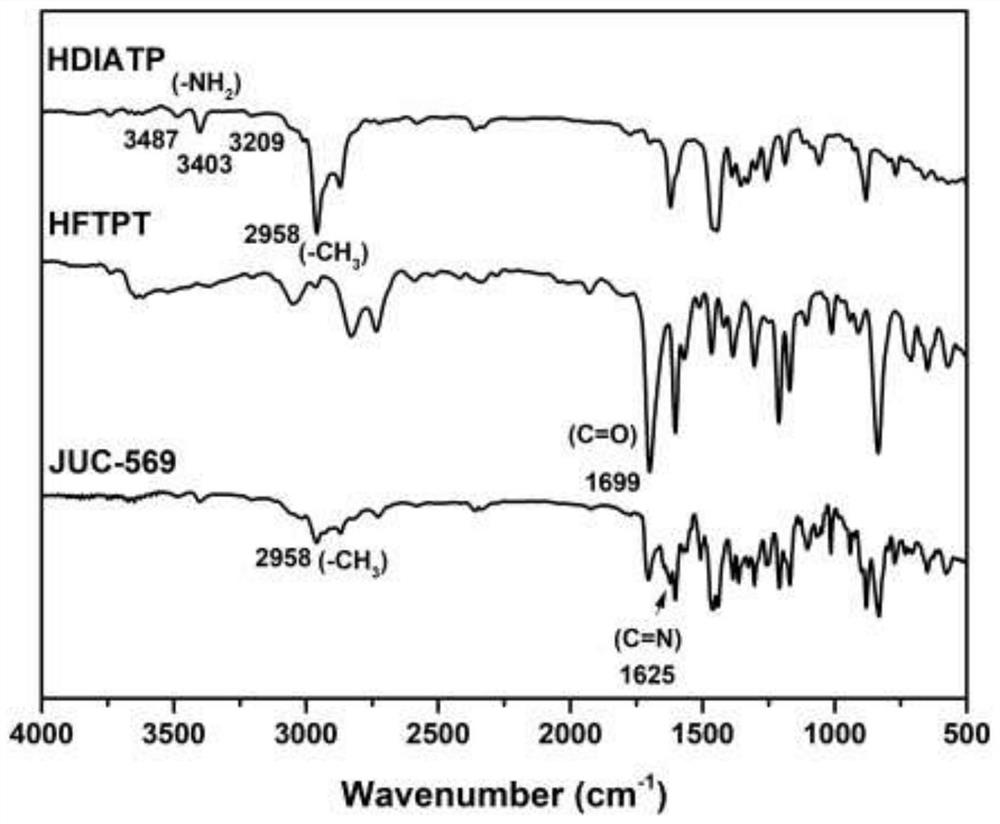
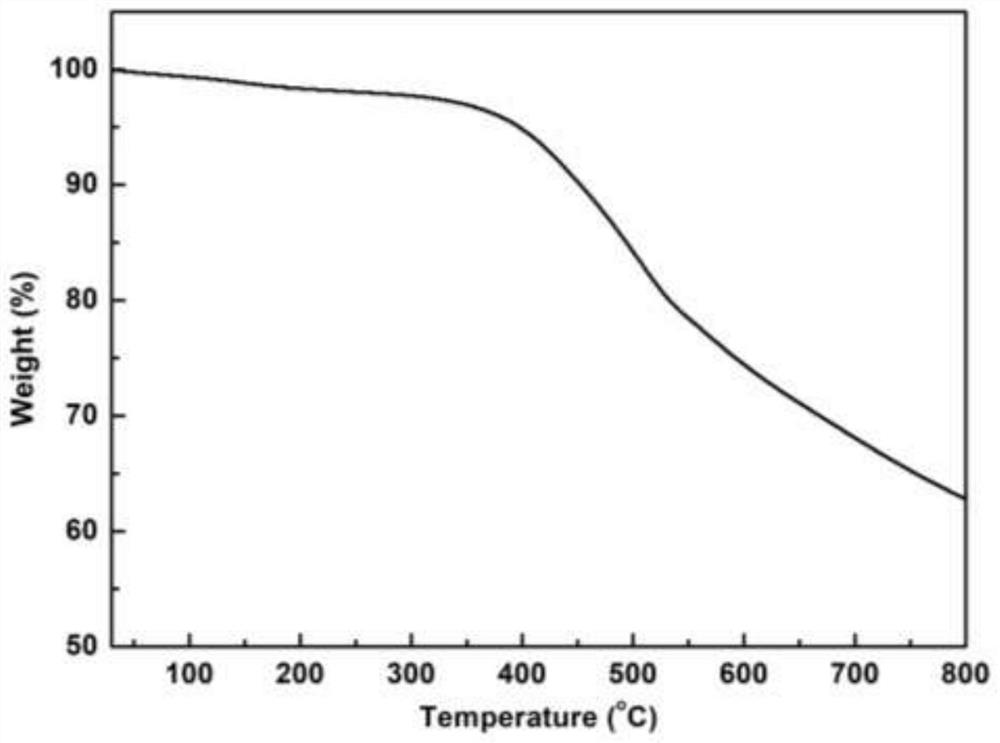
Three-dimensional covalent organic framework material based on amino derivatives of triptycene and preparation method thereof
A technology of covalent organic frameworks and amino derivatives, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of limited structure and restrict the development of three-dimensional COF materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A novel three-dimensional covalent organic framework based on triptycene-based amino derivatives, composed of 2,3,6,7,14,15-hexa(4-formylphenyl)triptycene and 2,3 ,6,7,14,15-hexa(2′,6′-diisopropyl-4′-amino) triptycene is an organic framework structure formed by condensation of building units through Schiff base reaction, and its structural formula is as follows:
[0039]
[0040] Further, the chemical structural formula of the 2,3,6,7,14,15-hexa(2′,6′-diisopropyl-4′-amino)triptycene (HDIATP) is as follows:
[0041]
[0042] A method for preparing a three-dimensional covalent organic framework material based on amino derivatives of triptycene, the specific steps are as follows:
[0043] (1) Synthesis of 2,3,6,7,14,15-hexabromotriptycene:
[0044]
[0045] Triptycene (1.00 g) and iron powder (80 mg) were dissolved in 60 mL of 1,2-dichloroethane. Then 1.32 mL of liquid bromine was slowly added to the flask. The mixture was then refluxed for 6 hours. After cool...
Embodiment 2
[0053] A method for preparing a three-dimensional covalent organic framework material based on amino derivatives of triptycene, the specific steps are as follows:
[0054] (1) Synthesis of 2,3,6,7,14,15-hexabromotriptycene:
[0055]
[0056] Triptycene (1.00 g) and iron powder (80 mg) were dissolved in 60 mL of 1,2-dichloroethane. Then 1.32 mL of liquid bromine was slowly added to the flask. The mixture was then refluxed for 6 hours. After cooling the reaction to 25 °C, the solvent and excess bromine were removed under reduced pressure. Residue with CHCl 2 After column separation as the developing solvent, use CHCl 3 Recrystallization, pure product as colorless, needle-like crystals, yield 77%.
[0057] (2) Synthesis of 2,3,6,7,14,15-hexa(2′,6′-diisopropyl-4′-amino)triptycene (Ⅱ):
[0058]
[0059] At 75°C, 2,3,6,7,14,15-hexabromotriptycene (500 mg), (N-(diphenylmethylene)-2,6-diisopropyl-4 -(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline) (3.50g), tetrakis(t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com