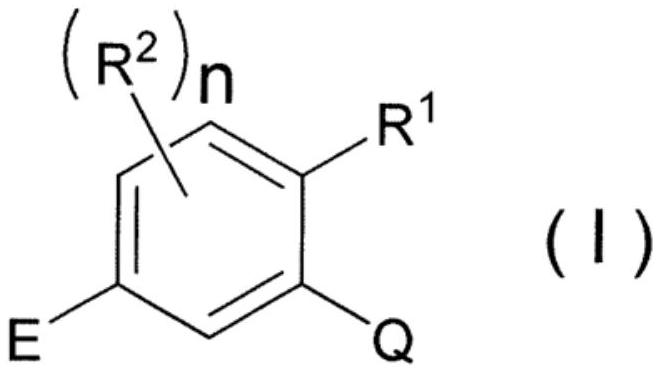
METHOD OF CONTROLLING SOYBEAN RUST FUNGUS THAT IS RESISTANT TO Qo INHIBITORS
A rust fungus and soybean technology, applied in the field of soybean rust fungus, can solve the problems of reduced affinity between fungicides and target enzymes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
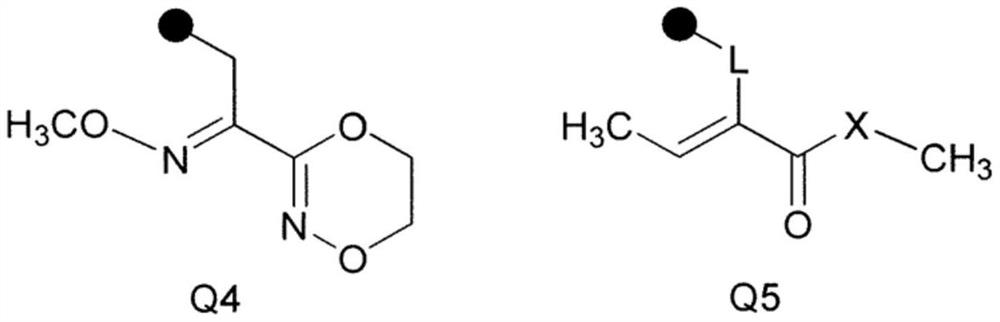
Image
Examples
Embodiment
[0554] The present invention will be described more specifically by showing production examples, preparation examples, and test examples below, but the present invention is not limited to these examples.
[0555] In this specification, Me means methyl, Et means ethyl, Pr means propyl, i-Pr means isopropyl, Bu means butyl, i-Bu means isobutyl, t-Bu means t-butyl, Pen means Pentyl, Hex represents hexyl, c-Pr represents cyclopropyl, c-Bu represents cyclobutyl, c-Pen represents cyclopentyl, c-Hex represents cyclohexyl, Ph represents phenyl. When Ph has a substituent, the substituent and the substituent site are put together before the symbol. For example, 3,4-Me 2 -Ph represents 3,4-dimethylphenyl.
[0556] Production examples of the present compound and the compound of the present invention are shown.
[0557] Reference manufacturing example 1
[0558] (3Z)-2-(5-bromo-2-methylphenoxy)-3-methoxymethyl acrylate (hereinafter referred to as intermediate) produced by the method de...
manufacture example 1
[0573] 0.50g of intermediate 1, 0.27g of 2-methylphenylboronic acid, [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) dichloromethane adduct 0.11 g. A mixture of 0.85 g of tripotassium phosphate, 15 mL of dimethoxyethane and 1 mL of water was stirred at 80° C. for 5 hours. After cooling the obtained mixture to room temperature, it was filtered. The filtrate was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The obtained residue was subjected to silica gel column chromatography (ethyl acetate:hexane=1:4) to obtain 0.42 g of the present compound 52 represented by the following formula.
[0574] [chemical formula 22]
[0575]
[0576] The present compound 52: 1 H-NMR (CDCl 3 )δ:7.28(1H,s),7.25-7.17(5H,m),6.87(1H,dd),6.69(1H,d),3.85(3H,s),3.70(3H,s),2.40(3H ,s),2.23(3H,s).
manufacture example 1-1
[0578] The compounds produced based on Production Example 1 and their physical properties are shown below.
[0579] In the compound shown in formula (1d), R 34 , R 35 , R 36 , R 37 , R 38 , R 39 and L is a compound of any combination described in [Table 1],
[0580] [chemical formula 23]
[0581]
[0582] [Table 1]
[0583] The compound R 34
R 35
R 36
R 37
R 38
R 39
L 53 F H H H H Me O 54 Cl H H H H Me O 55 H Cl H H H Me O 56 H H Cl H H Me O 57 H H H H H Cl O 58 F F H H H Me O 59 F H F H H Me O 60 F H H F H Me O 61 F H H H F Me O 62 F F F H H Me O 63 H F F F H Me O 87 H OPh H H H Me O
[0584] The present compound 53: 1 H-NMR (CDCl 3 )δ: 7.41-7.35 (1H, m), 7.33 (1H, s), 7.31-7.07 (5H, m), 6.93-6.91 (1H, m), 3.87 (3H, s), 3.71 (3H, s) , 2.40(3H,s).
[0585] The present compound 54: 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com